Redox Titration Between Mno4 And Fe2

Redox Titration Between Mno4 And Fe2 Youtube This video demonstrates the redox titration between potassium permanganate (kmno4) solution and iron (ii) (fe2 ) solution.mno4 5fe2 8h → 5fe3 mn2. 9.4: redox titrations is shared under a license and was authored, remixed, and or curated by libretexts. redox titration are here the titrant is an oxidizing or reducing agent. in contrast to acid base titrations, it is convenient for redox titrations to monitor the titration reaction’s potential ….
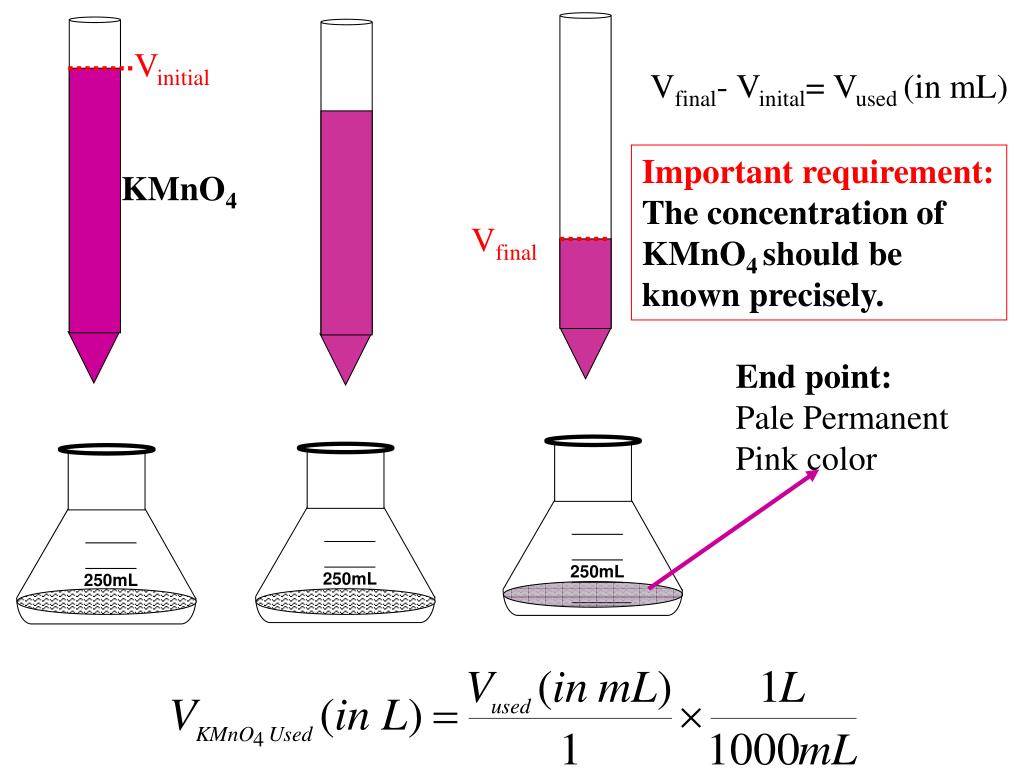
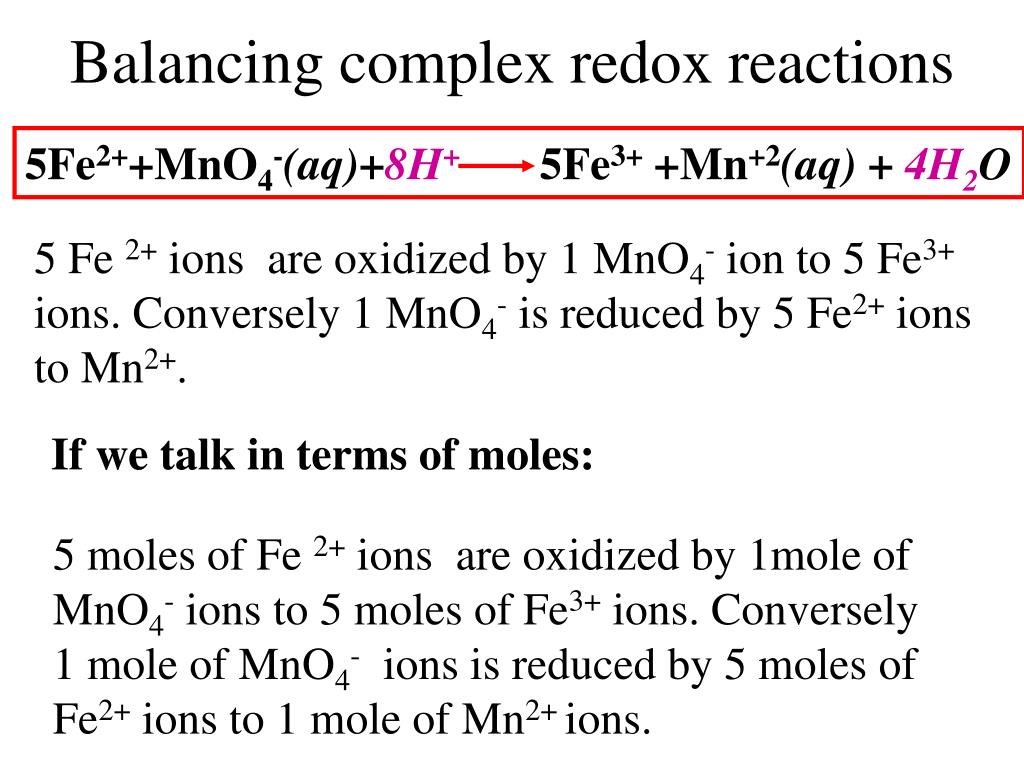
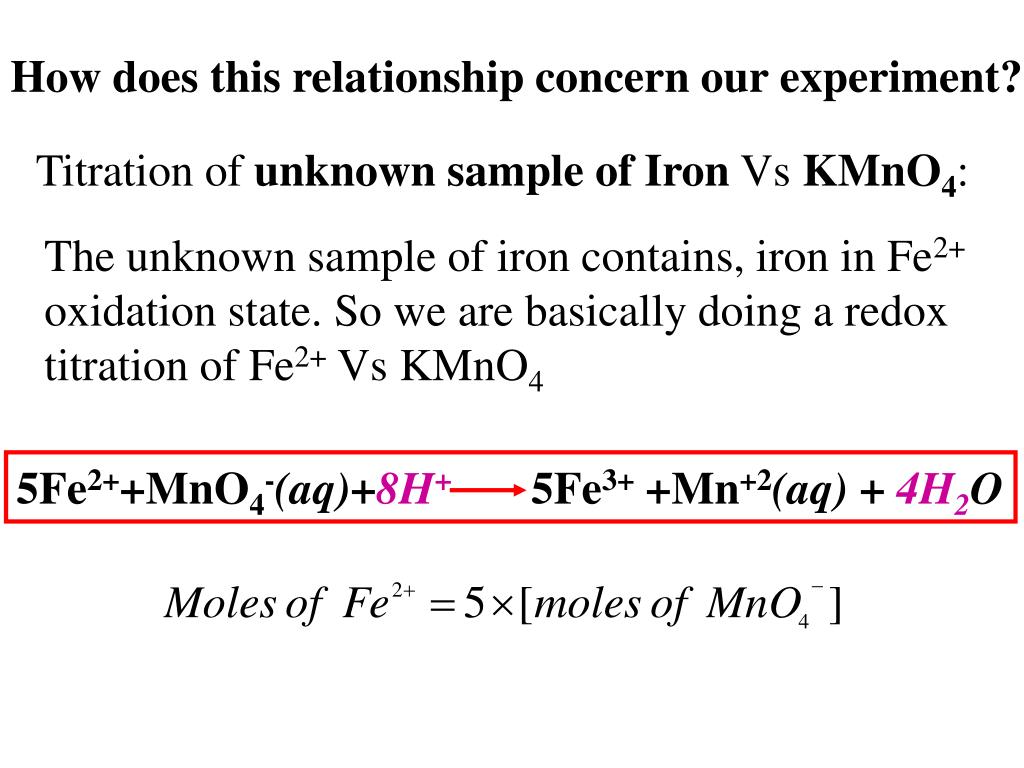
Ppt Redox Titration Powerpoint Presentation Id 431911 Redox titrations part 1 fe2 mno 4 this is a redox titration. it is specifically mentioned in the specification but it could be any redox reaction. mno 4 is just a common oxidising agent. mno 4 is usually added to the burette. no indicator is added as mno 4 is a deep purple colour, which acts as an indicator. when mno 4. Potassium manganate(vii) titrations. in these redox titrations the manganate(vii) is the oxidising agent and is reduced to mn 2 (aq); the iron is the reducing agent and is oxidised to fe 2 (aq) and the reaction mixture must be acidified, to excess acid is added to the iron(ii) ions before the reaction begins. This @theelkchemist a level short takes you through a 5 step method to solve a redox titration calculation problem involving potassium manganate (vii) & fe (. I think this is because $\ce{mno4 }$ ions have a very distinct colour. at the end of the reaction, the solution is colourless. i think that the $\ce{fe^3 }$ ions give the solution a brown colour, but it is in a very low concentration. the brown $\ce{fe^3 }$ ions are too few to give the whole solution a brown colour.
Ppt Redox Titration Powerpoint Presentation Free Download Id 431911 This @theelkchemist a level short takes you through a 5 step method to solve a redox titration calculation problem involving potassium manganate (vii) & fe (. I think this is because $\ce{mno4 }$ ions have a very distinct colour. at the end of the reaction, the solution is colourless. i think that the $\ce{fe^3 }$ ions give the solution a brown colour, but it is in a very low concentration. the brown $\ce{fe^3 }$ ions are too few to give the whole solution a brown colour. E = e ∘ inox inred ± 0.05916 n. this is the same approach we took in considering acid–base indicators and complexation indicators. a partial list of redox indicators is shown in table 9.4.2 . examples of an appropriate and an inappropriate indicator for the titration of fe 2 with ce 4 are shown in figure 9.4.5 . Redox titrations in general: determining the amount of reductant in a sample through titration with a strong oxidant. example: analysis of fe2 , titration with ce4 . ce4 e ↔ ce3 e° = 1.70 v fe3 e ↔ fe2 e° = 0.767 v therefore, titration reaction ce4 fe2 → ce3 fe3 is a highly favorable rxn and will effectively go to.
Ppt Redox Titration Powerpoint Presentation Id 431911 E = e ∘ inox inred ± 0.05916 n. this is the same approach we took in considering acid–base indicators and complexation indicators. a partial list of redox indicators is shown in table 9.4.2 . examples of an appropriate and an inappropriate indicator for the titration of fe 2 with ce 4 are shown in figure 9.4.5 . Redox titrations in general: determining the amount of reductant in a sample through titration with a strong oxidant. example: analysis of fe2 , titration with ce4 . ce4 e ↔ ce3 e° = 1.70 v fe3 e ↔ fe2 e° = 0.767 v therefore, titration reaction ce4 fe2 → ce3 fe3 is a highly favorable rxn and will effectively go to.

Comments are closed.