Regulatory Affairs In Pharmaceutical Industry Jli Blog
Regulatory Affairs For Pharma And Biotech Qbd Group Regulatory affairs is essential aspect of each industry for controlling safety and efficacy of products such as pharmaceutical products i.e. medicines, pesticides, agrochemicals, cosmetics, complementary and alternative medicines and medical devices. thus from the initiation of the trial, testing, packaging and marketing world over a regulatory. Jli main website; about jli; programs. medical writing; register now; regulatory affairs in pharmaceutical industry. posted may 27, 2019 may 27, 2019 admin. post.

Pharma Actd Dossiers Regulatory Affairs Regulatory Affairs James lind institute (jli) provides online program – professional diploma in pharmaceutical regulatory affairs and advance pg diploma in pharmacovigilance & regulatory affairs for better job opportunities in indian pharmaceutical industry. for more information please visit: jli.edu.in. regulatory affair professionals play a crucial role. A disconnected regulatory ecosystem coupled with an aggressive pharmaceutical industry falls short to fully clarify the roles and instill confidence in screening and approving capabilities of a new drug. banned drugs are still available in developing countries due to lack of physician awareness and law enforcement. Regulatory affairs. regulatory affairs specialists are expected to be extremely passionate regarding the safety and efficiency of drugs and medical devices in the health care industry. their interest for the sector must be deeply grounded into their function. this role demands expertise in a few distinct areas to be able to become successful. Pharmacovigilance and regulatory affairs – essential departments within the pharmaceutical industry posted on september 24, 2015 pharmacovigilance and regulatory affairs are an essential part of the larger health care industry.

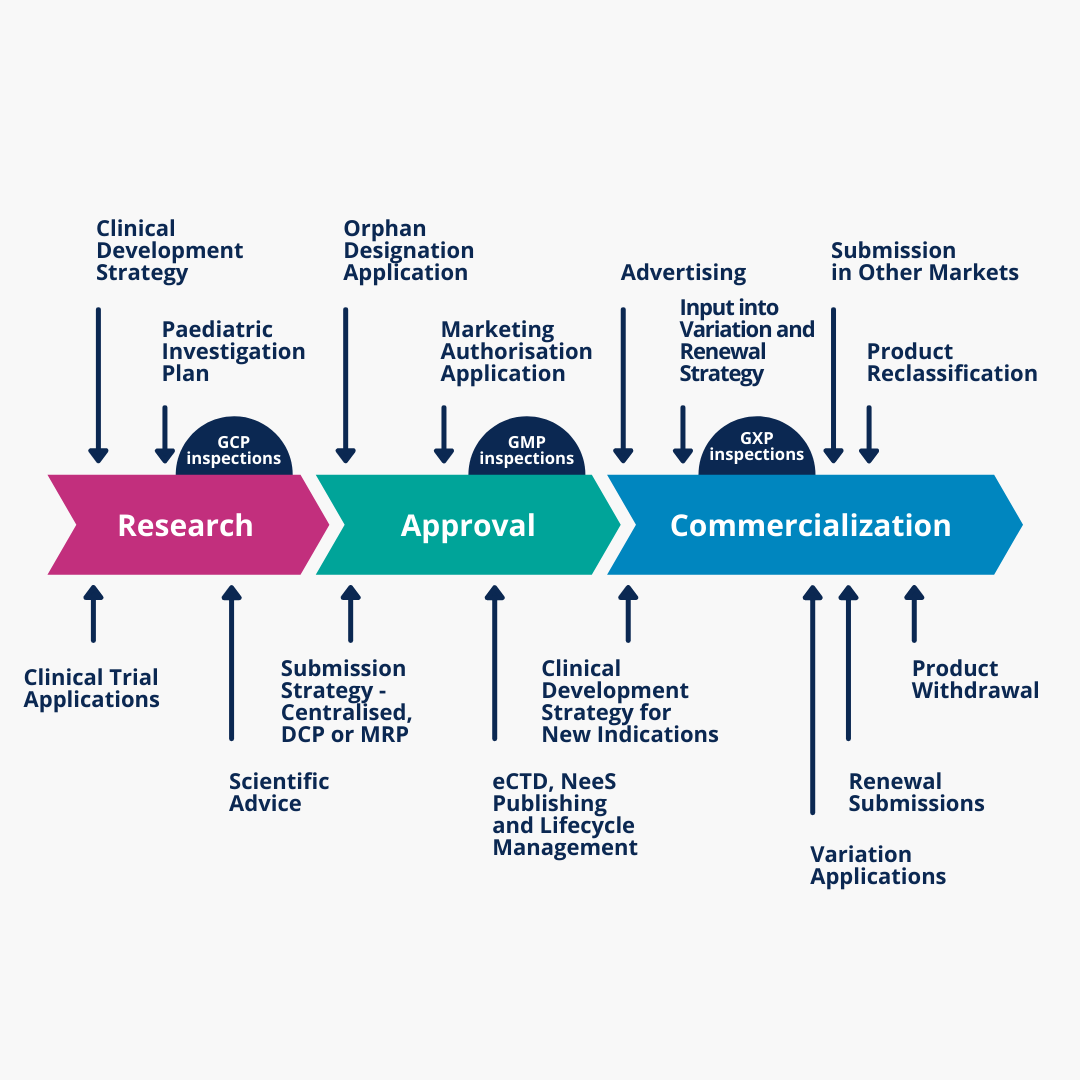
Regulatory Affairs Rls Pharma Services Regulatory affairs. regulatory affairs specialists are expected to be extremely passionate regarding the safety and efficiency of drugs and medical devices in the health care industry. their interest for the sector must be deeply grounded into their function. this role demands expertise in a few distinct areas to be able to become successful. Pharmacovigilance and regulatory affairs – essential departments within the pharmaceutical industry posted on september 24, 2015 pharmacovigilance and regulatory affairs are an essential part of the larger health care industry. December 8, 2021. regulatory affairs plays a key role in the pharmaceutical industry: from drug development to commercialization. learn more about the roles and functions that ra teams can provide in the lifecycle management of your pharmaceutical product. regulatory affairs plays a crucial role in the pharmaceutical industry, especially during. The curriculum for the program – advanced post graduate diploma in clinical research & regulatory affairs (apgdcr ra) is comprehensive, has been developed and evaluated by experienced pharmaceutical regulatory affairs professionals and is fully endorsed by the clinical research industry.

Regulatory Affairs It S Role In Pharmaceutical Industry December 8, 2021. regulatory affairs plays a key role in the pharmaceutical industry: from drug development to commercialization. learn more about the roles and functions that ra teams can provide in the lifecycle management of your pharmaceutical product. regulatory affairs plays a crucial role in the pharmaceutical industry, especially during. The curriculum for the program – advanced post graduate diploma in clinical research & regulatory affairs (apgdcr ra) is comprehensive, has been developed and evaluated by experienced pharmaceutical regulatory affairs professionals and is fully endorsed by the clinical research industry.

Comments are closed.