Responsibilities Of Investigator Ppt Video Online Download
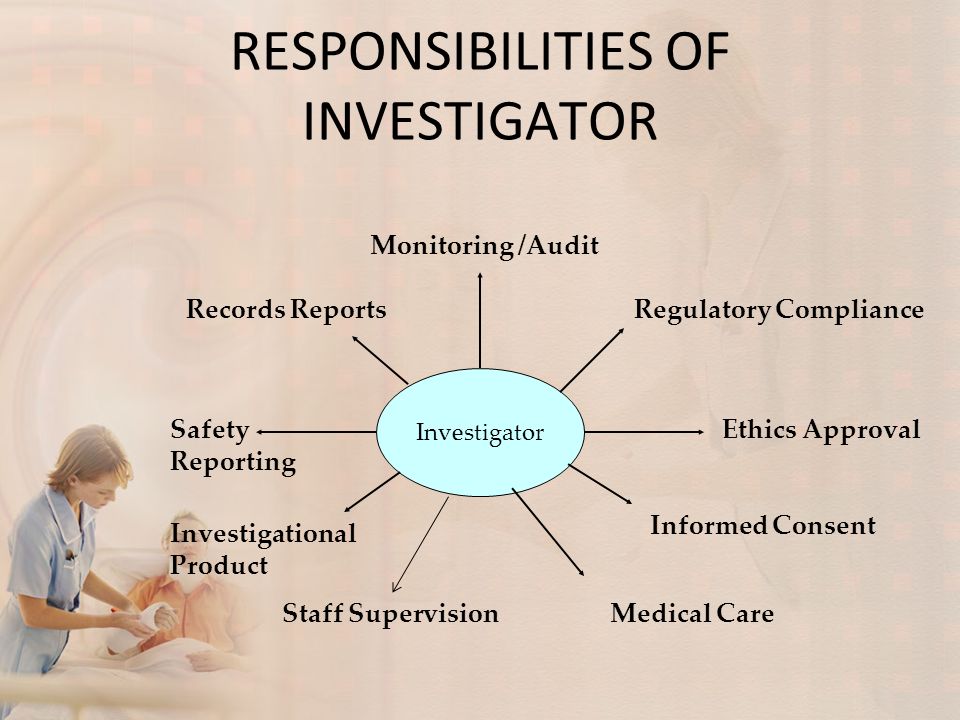
Responsibilities Of Investigator Ppt Video Online Download Presentation on theme: "responsibilities of investigator"— presentation transcript: 3 introduction an investigator is a person responsible for the conduct of the clinical trial at a trial site. the principal investigator is responsible for the collection, quality, recording, maintenance and retrieval of source data arising from the clinical. An investigator is responsible for leading the clinical trial team and ensuring compliance with regulations. key responsibilities include obtaining necessary approvals, ensuring safety of trial subjects, obtaining informed consent, accurately collecting and reporting data, and protecting subjects' rights and well being.

Responsibilities Of Investigator Ppt Video Online Download Roles and responsibilities of investigator [663] the document discusses the roles and responsibilities of investigators in clinical trials. it states that the principal investigator is responsible for leading the clinical trial team at a trial site and ensuring proper conduct of the trial. key responsibilities include being qualified to conduct. Investigator role and responsibilities. feb 15, 2020 •. 16 likes • 3,525 views. hsk college of pharmacy. follow. investigator: a person responsible for the conduct of the study at the trial site. investigator is a person responsible for the rights, health and welfare of the study subjects. read more. 1 of 36. Ich e6, 1.53. 5 sponsor’s responsibilities. quality assurance & quality control to ensure that trials are conducted & data are generated, documented & reported in compliance with the protocol, gcp & applicable regulatory requirements. to ensure direct access to trial related data by the sponsor or inspector (auditor). Investigator responsibilities • ensure that the clinical investigation is conducted according to the approved protocol and in compliance with all relevant sections of the u.s. code of federal regulations (cfr), ich gcp standards, and in country regulations and guidelines. • protect the rights, safety and welfare of subjects.

Ppt Investigator Responsibilities Powerpoint Presentation Free Ich e6, 1.53. 5 sponsor’s responsibilities. quality assurance & quality control to ensure that trials are conducted & data are generated, documented & reported in compliance with the protocol, gcp & applicable regulatory requirements. to ensure direct access to trial related data by the sponsor or inspector (auditor). Investigator responsibilities • ensure that the clinical investigation is conducted according to the approved protocol and in compliance with all relevant sections of the u.s. code of federal regulations (cfr), ich gcp standards, and in country regulations and guidelines. • protect the rights, safety and welfare of subjects. This guidance provides an overview of the responsibilities of a person who conducts a clinical investigation of a drug, biological product, or medical device (an investigator as defined in 21 cfr. Responsibilities of the pi • familiar with the background of the study (e.g., disease, management of the disease, side effects of treatment) • familiar with the study – the protocol document and study procedures • remember – reading is fundamental if you want to be an effective pi. some things to think about • how many errors could.

Ppt Investigator Responsibilities Powerpoint Presentation Free This guidance provides an overview of the responsibilities of a person who conducts a clinical investigation of a drug, biological product, or medical device (an investigator as defined in 21 cfr. Responsibilities of the pi • familiar with the background of the study (e.g., disease, management of the disease, side effects of treatment) • familiar with the study – the protocol document and study procedures • remember – reading is fundamental if you want to be an effective pi. some things to think about • how many errors could.

Comments are closed.