Results Of Cytotoxicity Test By Hemolysis With Blood Agar Using

Results Of Cytotoxicity Test By Hemolysis With Blood Agar Using We also found that testing using whole blood may result in significantly reduced hemolytic activity of test compounds (figure 6) in comparison to results obtained with washed erythrocytes (figure 7). the aim of this work is to convey increased awareness concerning these parameters, and to suggest a higher degree of standardization for the hemolysis assay. Results of cytotoxicity test by hemolysis with blood agar using microgel, gold nanorods and au nrs microgel composite samples, after 18 h at 37 • c and a 5% co 2 flow in the oven for cell culture.

Microbiology Hemolysis Blood Agar Youtube In vitro determination of hemolytic properties is a common and important method for preliminary evaluation of cytotoxicity of chemicals, drugs, or any blood contacting medical device or material. the method itself is relatively straightforward, however, protocols used in the literature vary substantially. this leads to significant difficulties both in interpreting and in comparing the obtained. 22.4: blood agar plates (bap). Blood agar plates and hemolysis protocols. An assay based on human blood hemolysis was developed to measure the remaining cytotoxicity of the cecm. the results from the hemolysis cytotoxicity assay were consistent with a standard live dead assay using ms1 endothelial cells incubated with the cecm. this study demonstrated an effective, reliable, and relatively inexpensive method for.

Blood Agar The Hemolysis Test Theory Results Youtube Blood agar plates and hemolysis protocols. An assay based on human blood hemolysis was developed to measure the remaining cytotoxicity of the cecm. the results from the hemolysis cytotoxicity assay were consistent with a standard live dead assay using ms1 endothelial cells incubated with the cecm. this study demonstrated an effective, reliable, and relatively inexpensive method for. Traditionally, hemolysin activity is measured on blood agar plates due to the simplicity of the assay. while this is telling, it cannot encapsulate the full story because s. aureus is known to behave differently in broth and on agar. furthermore, plate based assays are primarily semiquantitative and often a more accurate determination of. Blood agar is a general purpose, enriched medium often used to grow fastidious organisms and to differentiate bacteria based on their hemolytic properties. in the u.s., blood agar is usually prepared from tryptic soy agar or columbia agar base with 5% sheep blood.
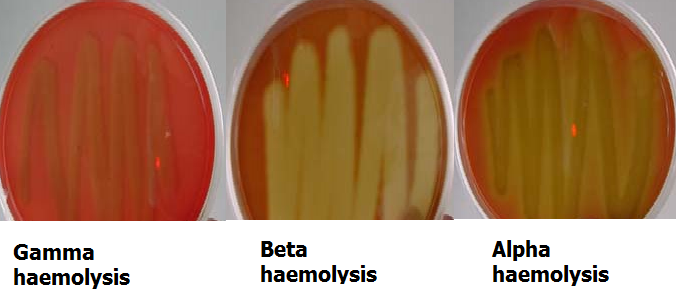
Blood Agar Haemolysis Test 1 Microbiology Resource Hub Traditionally, hemolysin activity is measured on blood agar plates due to the simplicity of the assay. while this is telling, it cannot encapsulate the full story because s. aureus is known to behave differently in broth and on agar. furthermore, plate based assays are primarily semiquantitative and often a more accurate determination of. Blood agar is a general purpose, enriched medium often used to grow fastidious organisms and to differentiate bacteria based on their hemolytic properties. in the u.s., blood agar is usually prepared from tryptic soy agar or columbia agar base with 5% sheep blood.
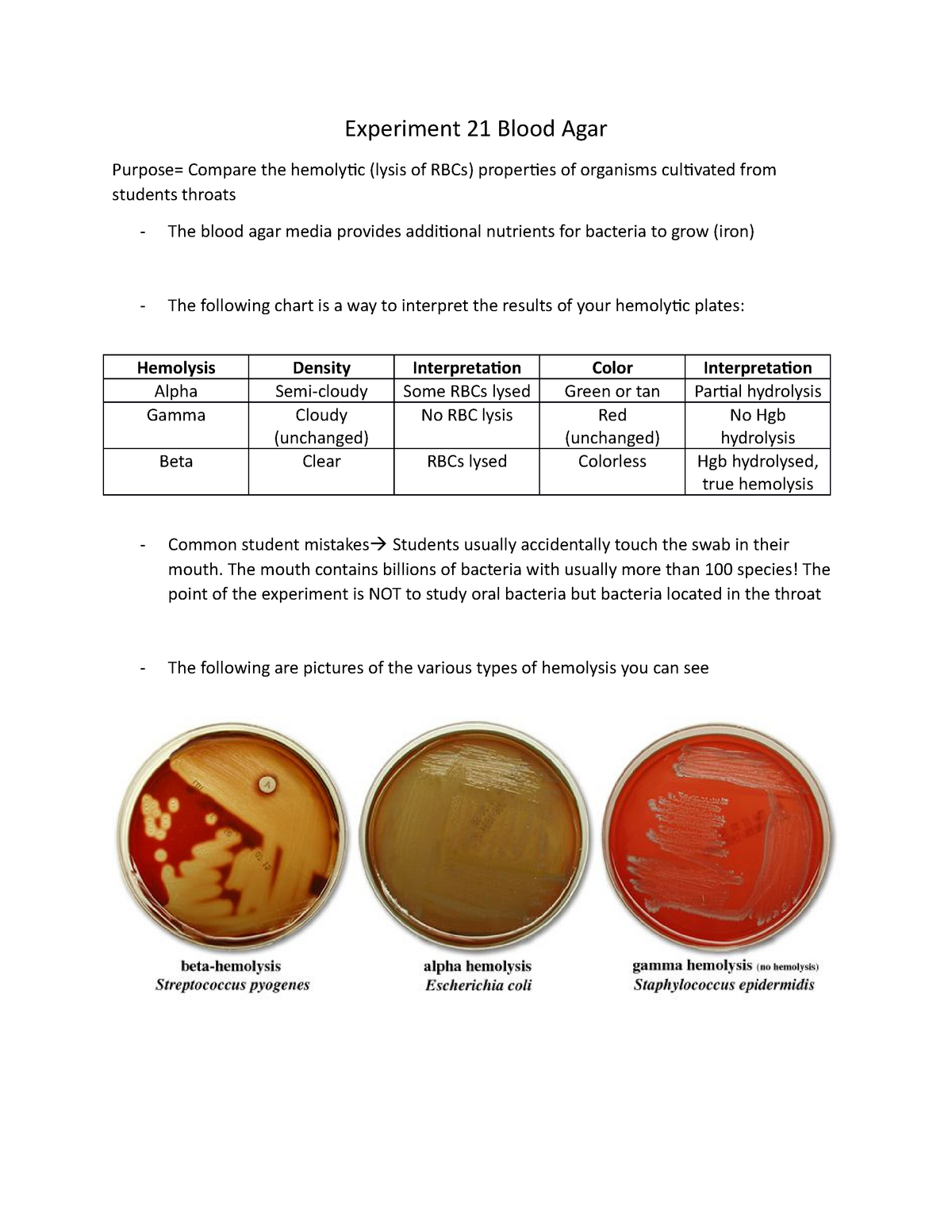
Experiment 21 Blood Agar Experiment 21 Blood Agar Purpose Compare

Comments are closed.