Risk Assessment In Clinical Laboratories
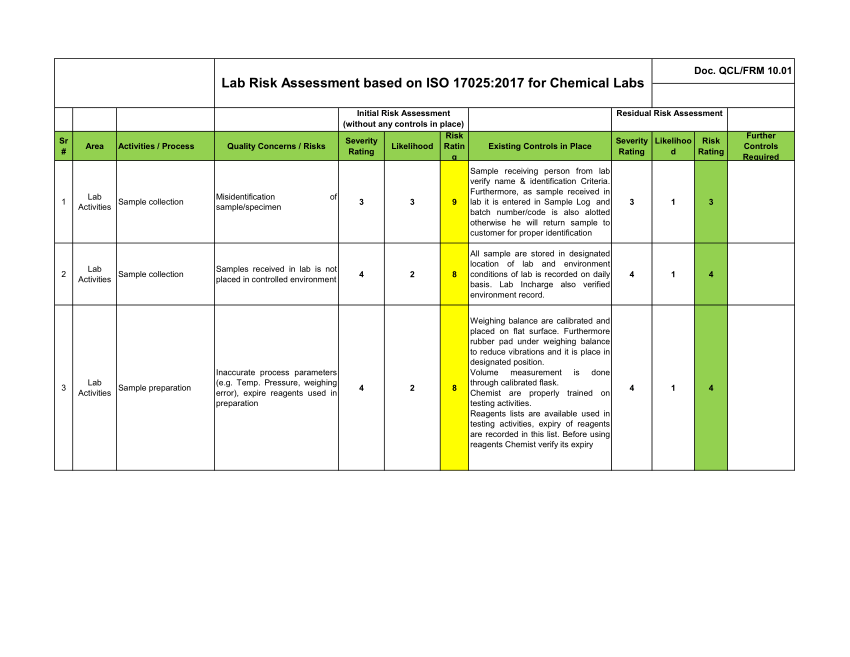
Pdf Iso 17025 Lab Risk Assessment The clinical laboratory improvement amendments of 1988 (clia) are the federal regulations that govern all us laboratory testing used for the “diagnosis, prevention, or treatment of any disease or impairment of the health of human beings.” 1 in 1992, when the initial amendments became effective, the minimum requirement for qc established for. We authors aimed to develop a practical tool for risk management in a clinical laboratory that contains five major cyclical steps: risk identification, quantification, prioritization, mitigation, and surveillance. the method for risk identification was based on a questionnaire that was formulated by evaluating five major components of.
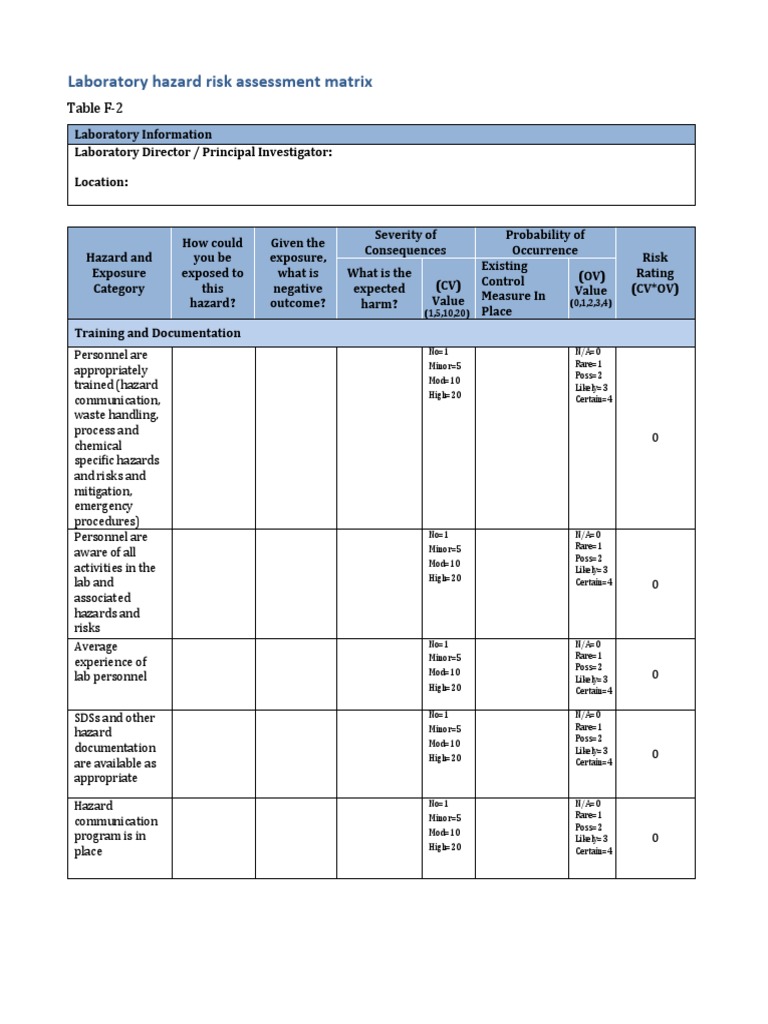
Table F 2 Laboratory Hazard Risk Assessment Matrix Template Pdf Biological risk assessments for clinical laboratories, july 2015 author: escott, shoolah h. (cdc osels lspppo) keywords: risk assessment, biosafety, biosecurity, clinical laboratory created date: 2 8 2018 10:51:35 am. The stanford laboratory risk assessment tool provides a framework for risk . assessment that maps onto the scientific method, melding with the process . researchers already use to answer scientific questions. this tool allows researchers to systematically identify and control . hazards to reduce risk of injuries and incidents. conduct a risk. Risk assessment. no laboratory test or process is without risk. moreover, because the laboratory testing process involves numerous steps, the number of potential errors can be large. it is therefore important to assess and prioritize risks and determine what level of risk is acceptable in the clinical laboratory. In general, risk assessments can be broken down into steps 1 2 in the figure above. the risk assessment should include considerations about the hazards (e.g., biological agent), the specific processes and procedures, existing control measures, the facility and testing environment, and the competency of the testing personnel.

Laboratory Risk Assessment Template Risk assessment. no laboratory test or process is without risk. moreover, because the laboratory testing process involves numerous steps, the number of potential errors can be large. it is therefore important to assess and prioritize risks and determine what level of risk is acceptable in the clinical laboratory. In general, risk assessments can be broken down into steps 1 2 in the figure above. the risk assessment should include considerations about the hazards (e.g., biological agent), the specific processes and procedures, existing control measures, the facility and testing environment, and the competency of the testing personnel. A key element of planning an experiment is assessing the hazards and potential risks associated with the chemicals and laboratory operations to be used. this chapter provides a practical guide for the trained laboratory personnel engaged in these activities. section 4.b introduces the sources of information for data on toxic, flammable, reactive, and explosive chemical substances. section 4.c. Overview. the who laboratory biosafety manual (lbm) has been in broad use at all levels of clinical and public health laboratories, and other biomedical sectors globally, serving as a de facto global standard that presents best practices and sets trends in biosafety. the lbm4 suite consists of one core document and 7 subject specific monographs.

Clinical Laboratory Risk Management In Clinical Laborator A key element of planning an experiment is assessing the hazards and potential risks associated with the chemicals and laboratory operations to be used. this chapter provides a practical guide for the trained laboratory personnel engaged in these activities. section 4.b introduces the sources of information for data on toxic, flammable, reactive, and explosive chemical substances. section 4.c. Overview. the who laboratory biosafety manual (lbm) has been in broad use at all levels of clinical and public health laboratories, and other biomedical sectors globally, serving as a de facto global standard that presents best practices and sets trends in biosafety. the lbm4 suite consists of one core document and 7 subject specific monographs.

Comments are closed.