Salt Analysis Formulas Complete List Formulae Sheet For Salt Analysis
Salt Analysis Formulas Complete List Formulae Sheet For Salt Analysis Ammonium nitrite. (nh 4 no 2 → n 2 2h 2 o) (ii) colourless gas with odour –. (a) nh 3 – turns red litmus blue and mercurous nitrate paper black. (b ) so 2 – smell of burning sulphur, turns acidified k 2 cr 2 o 7 paper green. (c) hcl pungent smell, white fumes with ammonia. (d) h 2 s – smell of rotten eggs, turns lead acetate paper. Step 6: now that the cation and the anion are identified, obtain the chemical formula of the salt by balancing the charges of the cation and anion. for example, if your cation is fe 3 and your anion is cl –, the chemical formula of the salt will be fecl 3. note: you can also identify the cation first and then move on to identifying the anion.

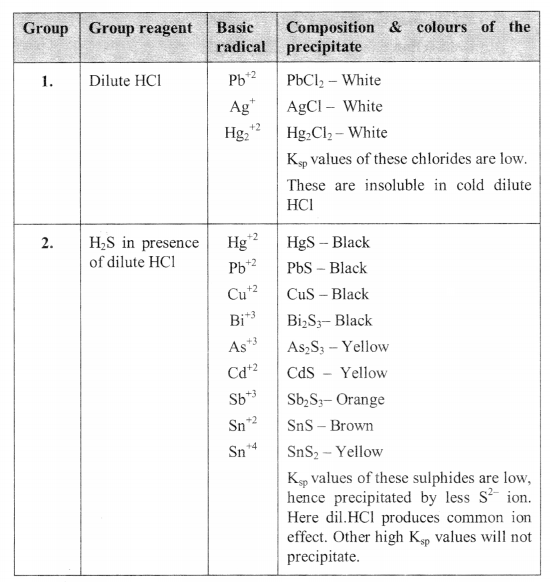
Chemistry Salt Analysis Class 12 Fe2 fe3 : note – ferrous salts are green in colour, ferric salts are brown in colour. 8 k2hgi4 9 original solution: salt acid water 1. if ferrous salt has been given, convert to ferric: os conc hno3 heat = brown ppt; then do reaction with group reagent 2. brown ppt hcl; then divide into two parts 1. potassium ferrocyanide. Success in salt analysis depends on a number of factors including: 1) adequate preparation before coming to lab; 2) the ability to read, follow, and efficiently execute all directions and procedures; 3) the ability to correctly interpret various test results. prior to coming to lab, study the material in the salt analysis section and these notes. This booklet describes the chemical analysis methods required for the evaluation and inspection of salts, which include sea salt, solar salt, rock salt, lake salt, and their equivalents. 2. general rules 2.1 reagents and apparatus (1) the reagents used in chemical analyses of salts shall, in principle, be of analytical grade, standardized by. Fe3 and your anion is cl , the chemical formula of the salt will be fecl 3. note: you can also identify the cation first and then move on to identifying the anion. salt analysis answer format (sample) a sample answer format for salt analysis is provided below. aim: to identify the acidic radical and the basic radical of the given inorganic salt.

Salt Analysis 12 Pdf Atoms Chemistry This booklet describes the chemical analysis methods required for the evaluation and inspection of salts, which include sea salt, solar salt, rock salt, lake salt, and their equivalents. 2. general rules 2.1 reagents and apparatus (1) the reagents used in chemical analyses of salts shall, in principle, be of analytical grade, standardized by. Fe3 and your anion is cl , the chemical formula of the salt will be fecl 3. note: you can also identify the cation first and then move on to identifying the anion. salt analysis answer format (sample) a sample answer format for salt analysis is provided below. aim: to identify the acidic radical and the basic radical of the given inorganic salt. Salt analysis. salt analysis, also known as qualitative inorganic analysis, is a branch of analytical chemistry aimed at identifying and classifying ions present in a sample. it involves a series of reactions with specific reagents that produce characteristic precipitates or color changes. these reactions form the basis of categorizing ions. A guide to common cations to help with salt analysis. the following guide provides information on common cations that can used to help with salt analysis. a guide to common anions in salt analysis. anion name formula molecular weight chloride cl 35.45 sulphate so4 2 96.07 carbonate co3 2 54.01 nitrate no3 62.00 phosphate po4 3 98.00.

Comments are closed.