Science4geeks Element Compound And Mixture

Science4geeks Element Compound And Mixture A mixture is made of two or more substances mixed but not chemically joined together. it can be separated into its components by physical means. it has the same properties as all its component substances and do not have a fixed composition of the substances. an example of mixture is air which consists of different gases such as nitrogen, oxygen. Close. compound a pure substance made from two or more elements which are chemically bonded in a fixed ratio. and. mixtures. close. mixture when two or more compounds or elements are present.

Science4geeks Element Compound And Mixture This chemistry video tutorial provides a basic introduction into the different types of matter such as elements, compounds, mixtures, and pure substances.che. What's the difference between a physical change and a chemical change? what are elements, compounds, pure substances, and mixtures? so many definitions to le. Ap chemistry quiz 1. 15 terms. robin00648. preview. homogeneous mixture. heterogeneous mixture. study with quizlet and memorize flashcards containing terms like compound, mixture, homogeneous mixture and more. Pure substances that are comprised of two or more elements are called compounds. compounds may be broken down by chemical changes to yield either elements or other compounds, or both. for example, mercury (ii) oxide, an orange, crystalline solid, can be broken down by heat into the elements mercury and oxygen (figure 4).
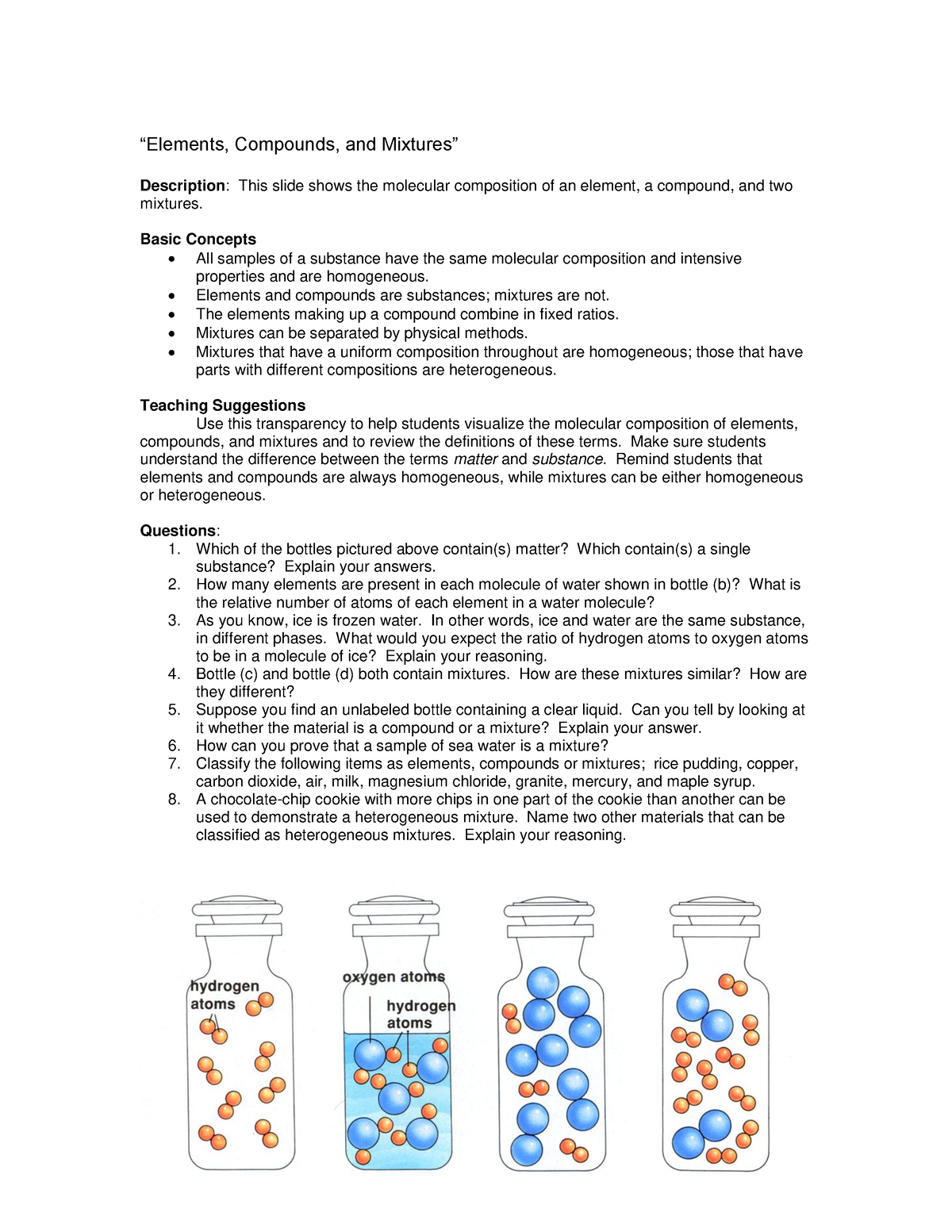
Element Compounds And Mixtures вђњelements Compounds And Mixtures Ap chemistry quiz 1. 15 terms. robin00648. preview. homogeneous mixture. heterogeneous mixture. study with quizlet and memorize flashcards containing terms like compound, mixture, homogeneous mixture and more. Pure substances that are comprised of two or more elements are called compounds. compounds may be broken down by chemical changes to yield either elements or other compounds, or both. for example, mercury (ii) oxide, an orange, crystalline solid, can be broken down by heat into the elements mercury and oxygen (figure 4). Matter can be broken down into two categories: pure substances and mixtures. pure substances are further broken down into elements and compounds. mixtures are physically combined structures that can be separated into their original components. a chemical substance is composed of one type of atom or molecule. a mixture is composed of different. Mixtures. microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). note that a mixture: consists of two or more different elements and or compounds physically intermingled, can be separated into its components by physical means, and. often retains many of the properties of its components.

Element Compound Or Mixture Card Sort Ks3 Beyond Matter can be broken down into two categories: pure substances and mixtures. pure substances are further broken down into elements and compounds. mixtures are physically combined structures that can be separated into their original components. a chemical substance is composed of one type of atom or molecule. a mixture is composed of different. Mixtures. microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). note that a mixture: consists of two or more different elements and or compounds physically intermingled, can be separated into its components by physical means, and. often retains many of the properties of its components.

Comments are closed.