Secrets Of Periodic Table How To Read Periodic Table ш шіш ш ш ш щ ш ш

Secrets Of Periodic Table How To Read Periodic Table ш шіш ш ш ш Download article. 1. read the periodic table from top left to bottom right. the elements are ordered by their atomic numbers, which increase as you move across and down the periodic table. the atomic number is how many protons the element’s atom possesses. Elements: a pure substance composed of a single atom with a unique atomic number. groups: the vertical column of the periodic table that signifies the number of valence electrons in an element. periods: the horizontal rows in the periodic table that signify the number of electron shells in an element. families: elements that have the same.
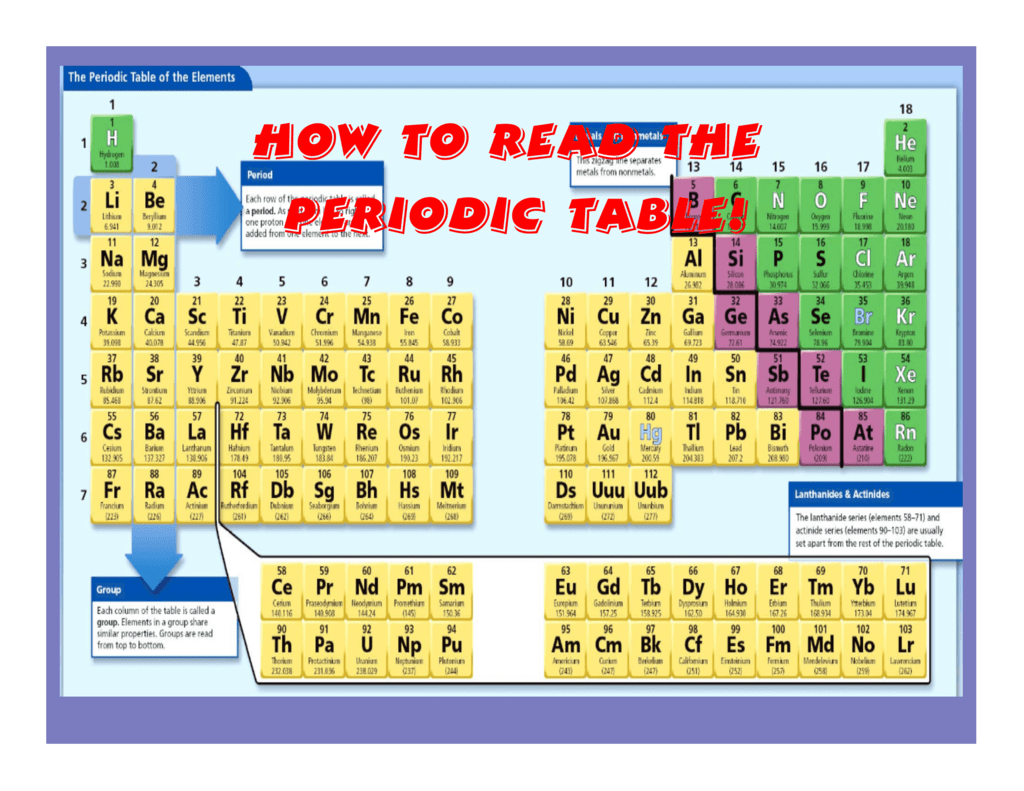
How To Read The Periodic Table вђ Theme Route 📌visit ‘periodic table with shell diagrams’ from here: knordslearning periodic table energy levels electrons shell 📌visit interactive periodic. E l e m e n t b o x e x a m p l e. carbon is located in period 2, group 14 of the periodic table. it is the lightest nonmetal. let us study the information in the element box. carbon has 6 protons and 6 electrons. hence, its atomic number is 6. the elemental symbol of carbon is c, the first letter of its english name. Sodium (na), the 11th element in the periodic table, is commonly found as a soft metal that reacts violently with water. chlorine (cl), the 17th element in the periodic table, most commonly exists as a yellowish green toxic gas. but when one atom of sodium combines with one atom of chlorine, you get sodium chloride (nacl), commonly known as. Periodic tables generally display two numbers with each element. the smaller number is the atomic number. this is the number of protons, which is unique to each element and doesn’t change. the larger number is the relative atomic mass of an element – the higher the number, the greater its mass. more elements could be added to the periodic.
How To Read Periodic Table Elements Sodium (na), the 11th element in the periodic table, is commonly found as a soft metal that reacts violently with water. chlorine (cl), the 17th element in the periodic table, most commonly exists as a yellowish green toxic gas. but when one atom of sodium combines with one atom of chlorine, you get sodium chloride (nacl), commonly known as. Periodic tables generally display two numbers with each element. the smaller number is the atomic number. this is the number of protons, which is unique to each element and doesn’t change. the larger number is the relative atomic mass of an element – the higher the number, the greater its mass. more elements could be added to the periodic. To do this, round up the atomic weight to the nearest whole number to find the mass number, then subtract the number of protons. the resulting number is the number of neutrons in the atom. wikihow quick video on how to read the periodic table. the periodic table can seem a little daunting at first. For example, memorize all of the elements up to helium, then memorize elements from sodium to argon, and so one. next, combine two of your sets. eventually, work up to all 118 elements. use the numbers and group colors as prompts initially. finally, just recite the entire periodic table without consulting the cards.

Comments are closed.