Should Your Research Site Even Do Phase 4 Trialsо
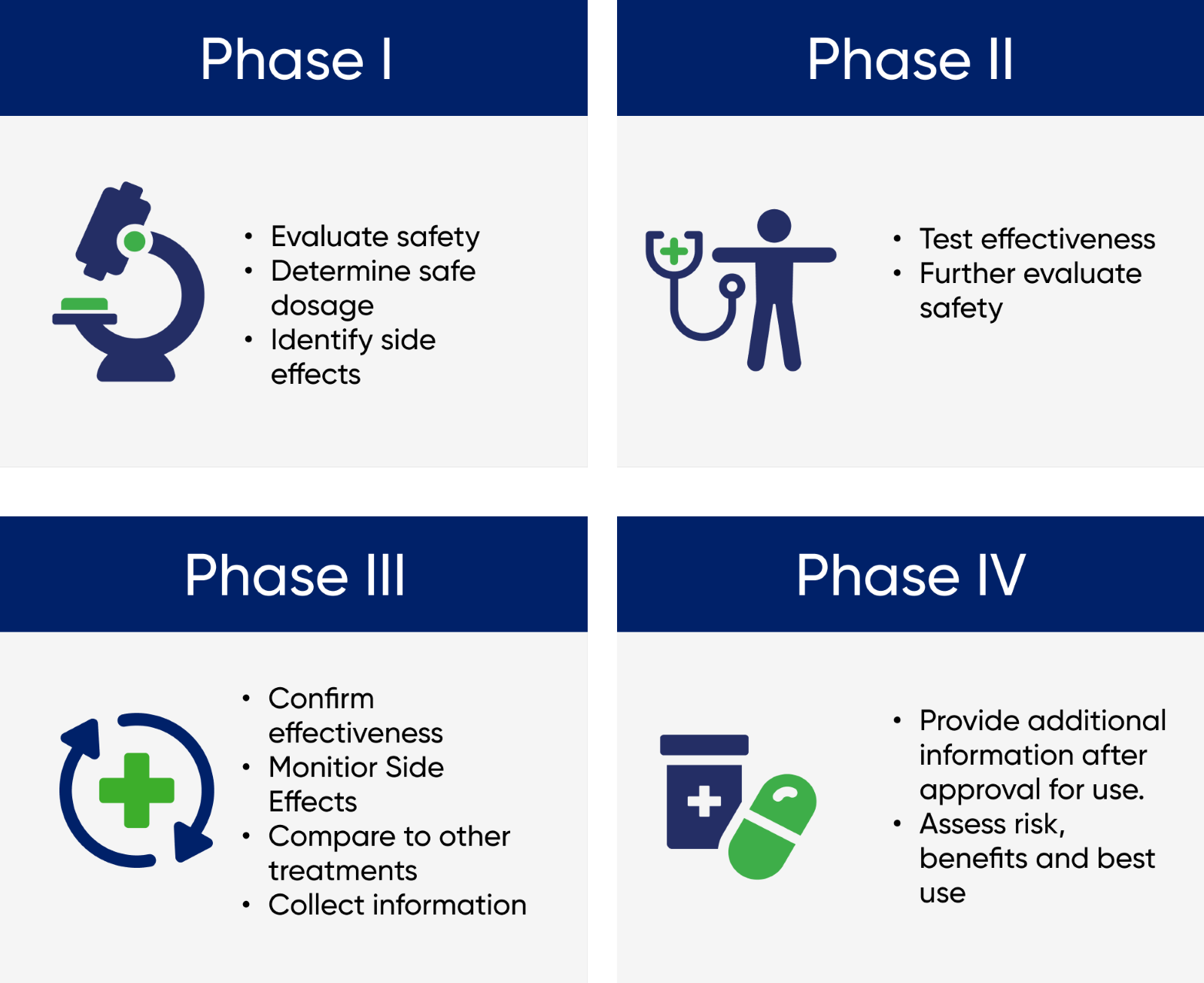
Participants Aotearoa Clinical Trials Auckland New Zealand The main objective of the phase 4 trial is to check the drug's performance in real life scenarios, to study the long term risks and benefits of using the drug and to discover any rare side effects. Phase 4 clinical trials are aimed to provide broader information about the efficacy and safety of the new medication or intervention in large numbers of patients. this phase is meant to assess the drug’s long term effects. less common adverse events may be detected in these circumstances. this phase involves thousands of people.

Question 4 Following Successful Phase 3 Clinical Chegg The four phases of clinical trials. early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. later phases (phase 3 and 4) compare the treatment to current standard treatments. in a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Phase 4 clinical trials. after the fda approves a treatment, it may be studied more in a phase 4 trial. we do not do phase 4 clinical trials at msk. phase 4 trials assess the side effects, risks, and benefits of a drug or other therapy. they test it over a long period of time and in a larger number of people than in phase 3 trials.

Review Approach To Support The Discussion On Future Research Directions Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Phase 4 clinical trials. after the fda approves a treatment, it may be studied more in a phase 4 trial. we do not do phase 4 clinical trials at msk. phase 4 trials assess the side effects, risks, and benefits of a drug or other therapy. they test it over a long period of time and in a larger number of people than in phase 3 trials. Later phase trials aim to test whether a new treatment is better than existing treatments. there are 3 main phases of clinical trials – phases 1 to 3. phase 1 trials are the earliest phase trials and phase 3 are later phase trials. some trials have an earlier stage called phase 0, and there are some phase 4 trials done after a drug has been. Watch this video to learn about the three phases of clinical trials. clinical research phase studies. phase 1. study participants: 20 to 100 healthy volunteers or people with the disease condition.
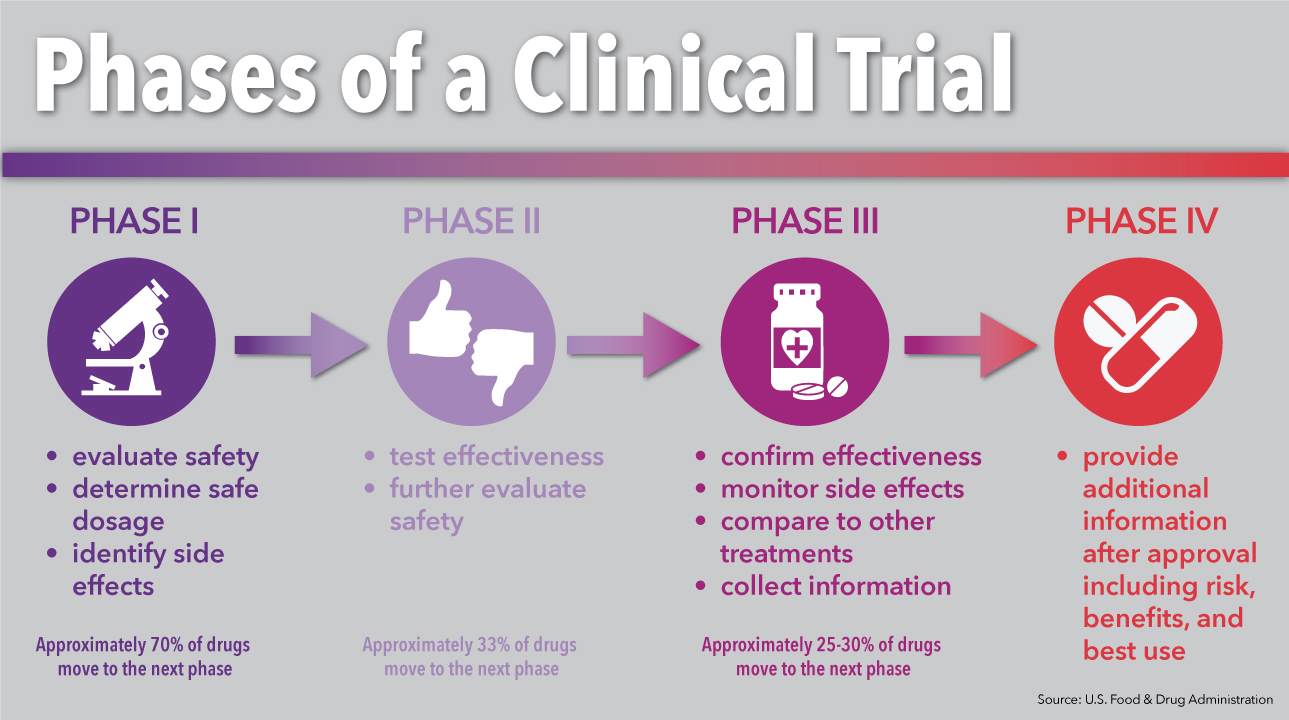
مطالعات بالینی یا کارآزمایی بالینی چیست وبلاگ شبکه مترجمین ایران Later phase trials aim to test whether a new treatment is better than existing treatments. there are 3 main phases of clinical trials – phases 1 to 3. phase 1 trials are the earliest phase trials and phase 3 are later phase trials. some trials have an earlier stage called phase 0, and there are some phase 4 trials done after a drug has been. Watch this video to learn about the three phases of clinical trials. clinical research phase studies. phase 1. study participants: 20 to 100 healthy volunteers or people with the disease condition.

Comments are closed.