Sickle Cell Disease Sangamo
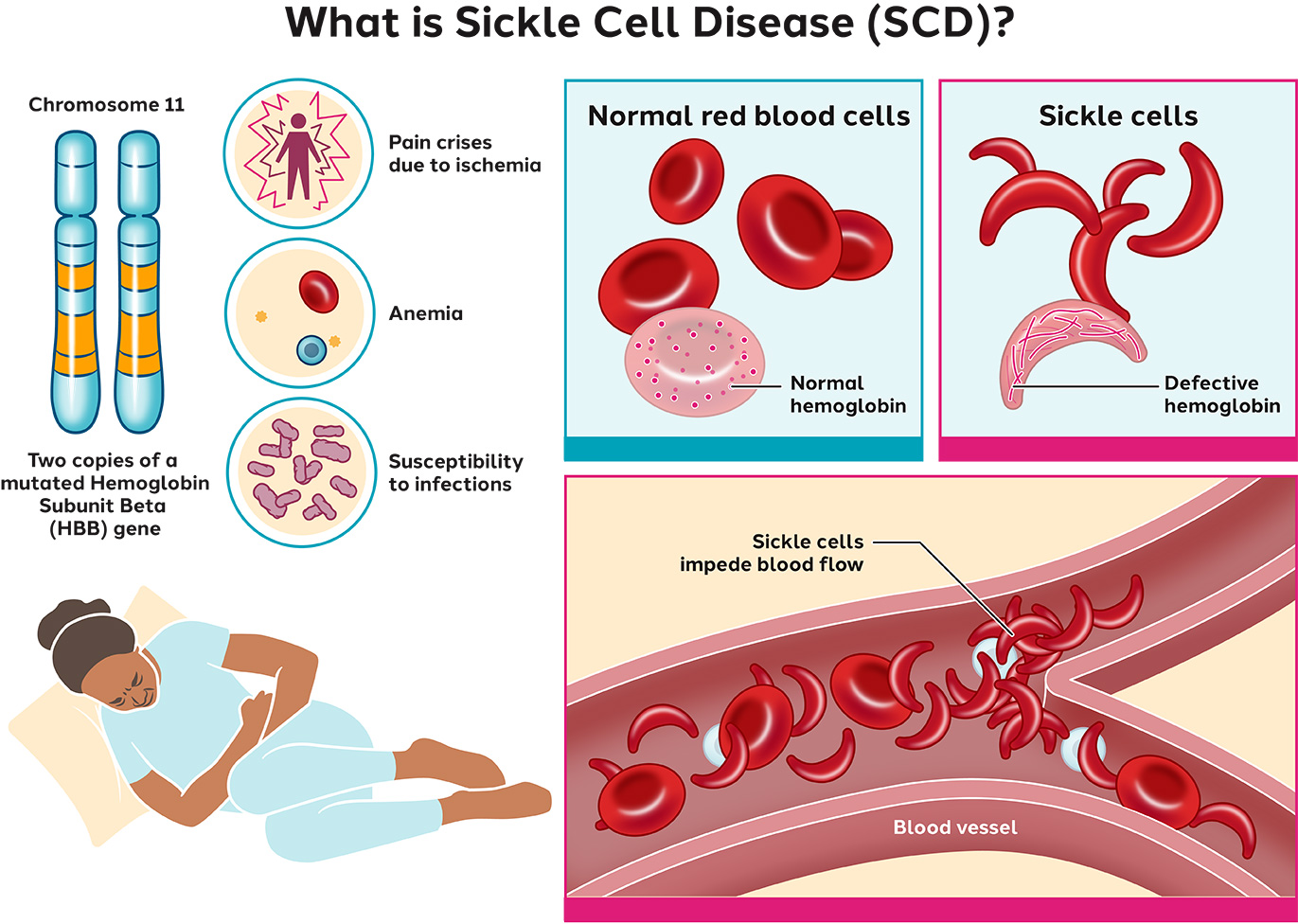
Sickle Cell Disease Sangamo Sickle cell disease (scd) is a blood disorder caused by a mutation in the hemoglobin gene, resulting in distorted red blood cells (sickle cells). these sickle cells either die early or cause blockages in small blood vessels. scd’s main symptom is agonizing pain – either chronic or episodic – due to ischemia, a restriction in the blood. These forward looking statements include, without limitation, statements regarding the therapeutic potential of sar445136, including its potential to relieve people living with sickle cell disease of some of their most challenging symptoms, the anticipated completion of the precizn 1 study and dosing of the final patients in the precizn 1 study.

Sangamo Announces Transition Of Sar445136 Sickle Cell Disease Program Sickle cell pipeline narrows as gene therapy developers rethink research plans. graphite bio and sangamo are stopping work on their respective gene therapies, while intellia revealed partner novartis has ended development of its genetic treatment for the blood disease. published feb. 23, 2023. ned pagliarulo lead editor. These forward looking statements include, without limitation, statements regarding the therapeutic potential of sar445136, including its potential clinical benefit to patients with sickle cell disease and its potential as an alternative to the standard of care for patients with sickle cell disease, and other statements that are not historical. •sickle cell disease (scd) is caused by pathologic variants in both alleles of the β globin gene •elevated fetal hemoglobin (hbf) levels inhibit sickle cell formation, ameliorate symptoms, and improve survival in patients with scd •bivv003: novel product candidate comprising autologous cd34 hematopoietic stem and. Sangamo said it remains committed to progressing this program and believe sar445136 has the potential to relieve people living with sickle cell disease of some of their most challenging symptoms.

Sangamo S Sar445136 Shows Tolerability And Sustained Effects In Sickle •sickle cell disease (scd) is caused by pathologic variants in both alleles of the β globin gene •elevated fetal hemoglobin (hbf) levels inhibit sickle cell formation, ameliorate symptoms, and improve survival in patients with scd •bivv003: novel product candidate comprising autologous cd34 hematopoietic stem and. Sangamo said it remains committed to progressing this program and believe sar445136 has the potential to relieve people living with sickle cell disease of some of their most challenging symptoms. Sangamo announces ema releases details supporting orphan designation for bivv003 for the treatment of sickle cell disease march 17, 2021 04:30 pm eastern daylight time. An ex vivo gene edited cell therapy for sickle cell disease (scd) being developed by sangamo therapeutics and sanofi has generated positive early phase i ii results in three patients—data that.

Comments are closed.