Solutions Percent By Mass And Volume

Solutions Percent By Mass And Volume Youtube Percent means per 100 parts, where for solutions, part refers to a measure of mass (μg, mg, g, kg, etc.) or volume (μl, ml, l, etc.). in percent solutions, the amount (weight or volume) of a solute is expressed as a percentage of the total solution weight or volume. percent solutions can take the form of weight volume % (wt vol % or w v. Solutions, percent by mass and volume.chemistry lecture #76.for a pdf transcript of this lecture, go to richardlouie.

Percent By Volume Definition And Example V V The percentage concentration of any solution is most commonly expressed as mass percent: mass % of any component of the solution =. (mass of the component in the solution total mass of the solution) x 100. other methods are: volume percentage: volume % of a component =. (volume of the component total volume of the solution) x 100. If a solution is made by adding 40ml 40 ml of ethanol to 200ml 200 ml of water, the percent by volume is: percent by volume = volume of solute volume of solution × 100% = 40ml ethanol 240ml solution × 100% = 16.7%ethanol percent by volume = volume of solute volume of solution × 100 % = 40 ml ethanol 240 ml solution × 100 % = 16.7 % ethanol. Notice that it was necessary to subtract the mass of the nacl (150 g) from the mass of solution (3000 g) to calculate the mass of the water that would need to be added. volume percent. the percentage of solute in a solution can more easily be determined by volume when the solute and solvent are both liquids. Mass percent mass percent = msolute (g) msolution (g) m s o l u t e ( g) m s o l u t i o n ( g) × 100 100. because a solution is comprised of both a solute and a solvent, the mass of a solution, as a whole, is equal to the sum of the masses of the solute and the solvent that it contains. therefore, the following equation can also be used to.
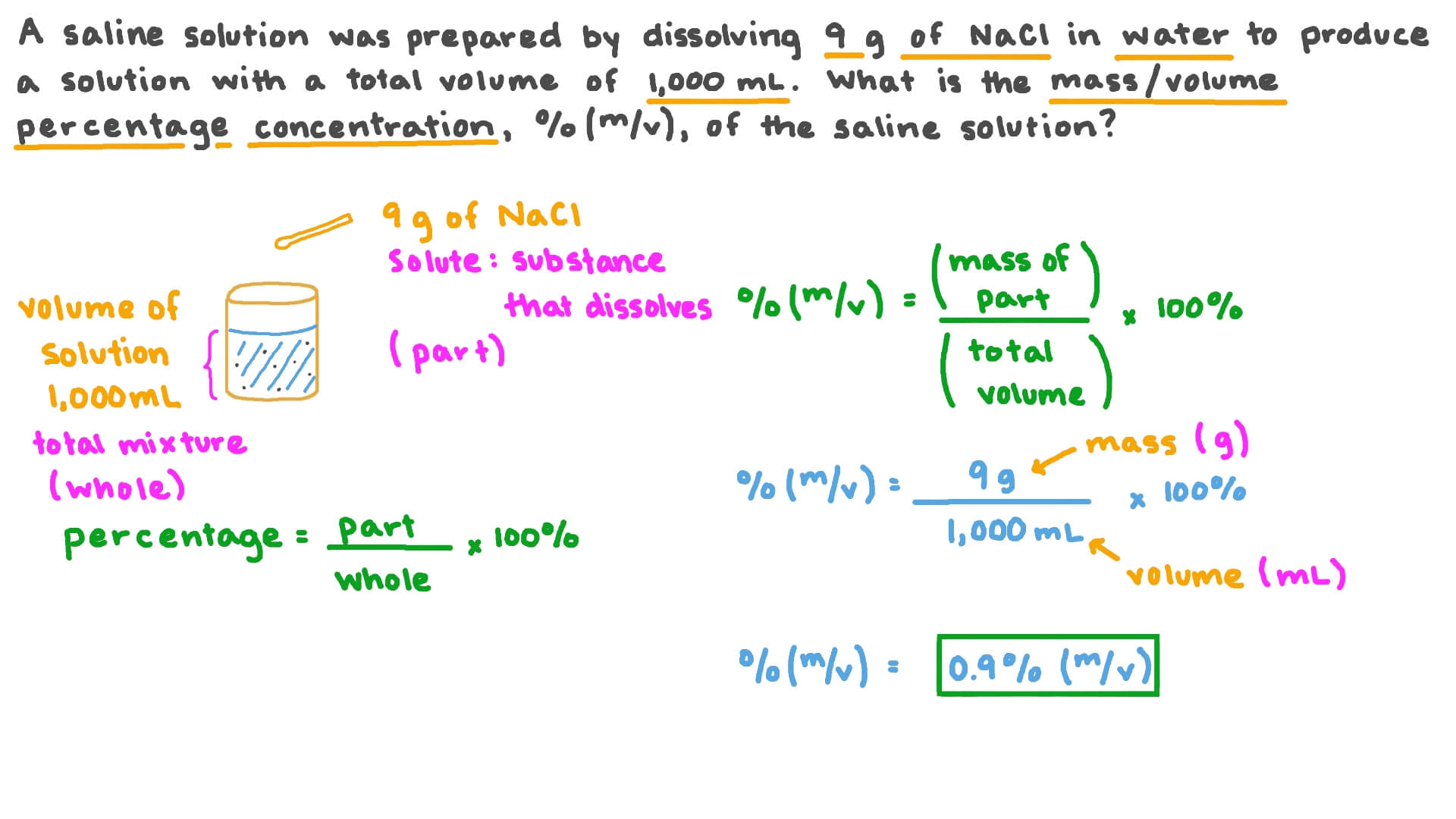
Question Video Calculating The Mass By Volume Percentage Concentration Notice that it was necessary to subtract the mass of the nacl (150 g) from the mass of solution (3000 g) to calculate the mass of the water that would need to be added. volume percent. the percentage of solute in a solution can more easily be determined by volume when the solute and solvent are both liquids. Mass percent mass percent = msolute (g) msolution (g) m s o l u t e ( g) m s o l u t i o n ( g) × 100 100. because a solution is comprised of both a solute and a solvent, the mass of a solution, as a whole, is equal to the sum of the masses of the solute and the solvent that it contains. therefore, the following equation can also be used to. A mass volume percent is a ratio of a solute’s mass to the solution’s volume expressed as a percentage. the specific units used for solute mass and solution volume may vary, depending on the solution. for example, physiological saline solution, used to prepare intravenous fluids, has a concentration of 0.9% mass volume (m v), indicating. The mass of the solute in the solution. the mass of the solution. use the following equation to calculate percent by mass: top molarity. molarity tells us the number of moles of solute in exactly one liter of a solution. (note that molarity is spelled with an "r" and is represented by a capital m.) we need two pieces of information to calculate.

Comments are closed.