Solved 11 A Write The Chemical Equation For The Net Ionic Reaction
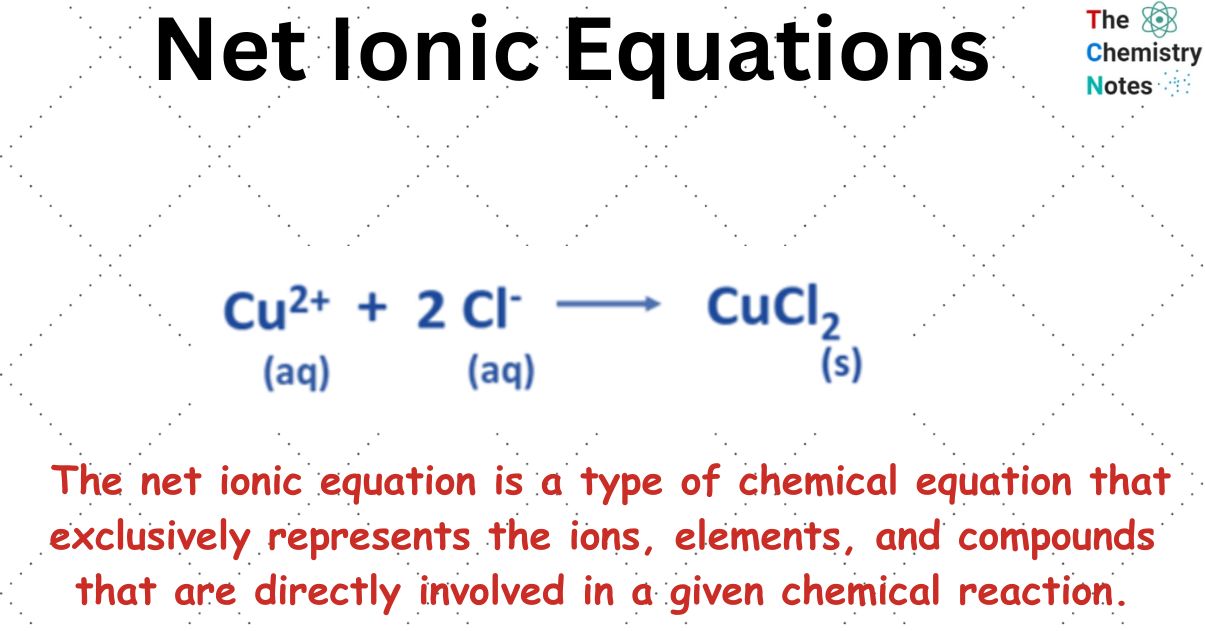
Net Ionic Equations Definition Examples The equation can now be written without the spectator ions: ag (aq) cl− (aq) → agcl(s) ag (a q) cl − (a q) → agcl (s) the net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. notice that in writing the net ionic equation, the positively. Exercise 8.11.1 8.11. 1. write the chemical equation that represents the dissociation of (nh 4) 2 s. answer. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the complete ionic formula. a complete ionic equation is a chemical equation in.

Solved For The Following Chemical Reaction Write The Net Chegg The simplified net ionic equation is, ch3co2h(aq) oh–(aq) –> ch3co2–(aq) h2o(l) example 2. write the net ionic equation that represents the reaction of aqueous solution of sodium chromate and lead (ll) nitrate which react to form a yellow precipitate of lead (ll) chromate and aqueous solution of sodium nitrate. solution. This chemistry video tutorial explains how to write net ionic equations. it explains how to predict the products of double replacement reactions and acid ba. 1. know the difference between molecular and ionic compounds. the first step in writing a net ionic equation is identifying the ionic compounds of the reaction. ionic compounds are those that will ionize in an aqueous solution and have a charge. [2] molecular compounds are compounds that never have a charge. The equation can now be written without the spectator ions: ag (aq) cl− (aq) → agcl(s) ag (a q) cl − (a q) → agcl (s) the net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. notice that in writing the net ionic equation, the positively.

Solved 11 Write The Net Ionic Equation For The Following Chegg 1. know the difference between molecular and ionic compounds. the first step in writing a net ionic equation is identifying the ionic compounds of the reaction. ionic compounds are those that will ionize in an aqueous solution and have a charge. [2] molecular compounds are compounds that never have a charge. The equation can now be written without the spectator ions: ag (aq) cl− (aq) → agcl(s) ag (a q) cl − (a q) → agcl (s) the net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. notice that in writing the net ionic equation, the positively. The first step to writing a net ionic equation is balancing the chemical equation present. let’s use the reaction between sodium chloride and silver nitrate as an example. next, we write the chemical equation as a complete ionic equation. this means that we separate each molecule into its ion form. (note that only aqueous compounds can be. There are different ways to write equations for chemical reactions. some of the most common are unbalanced equations, which indicate the species involved; balanced chemical equations, which indicate number and type of species; molecular equations, which express compounds as molecules instead of component ions; and net ionic equations, which only deal with the species that contribute to a reaction.

How To Write A Net Ionic Equation 10 Steps With Pictures The first step to writing a net ionic equation is balancing the chemical equation present. let’s use the reaction between sodium chloride and silver nitrate as an example. next, we write the chemical equation as a complete ionic equation. this means that we separate each molecule into its ion form. (note that only aqueous compounds can be. There are different ways to write equations for chemical reactions. some of the most common are unbalanced equations, which indicate the species involved; balanced chemical equations, which indicate number and type of species; molecular equations, which express compounds as molecules instead of component ions; and net ionic equations, which only deal with the species that contribute to a reaction.

How To Write Net Ionic Equations Method Examples

Comments are closed.