Solved Compare The Solubility Of Iron Ii п їsulfide In Eachођ

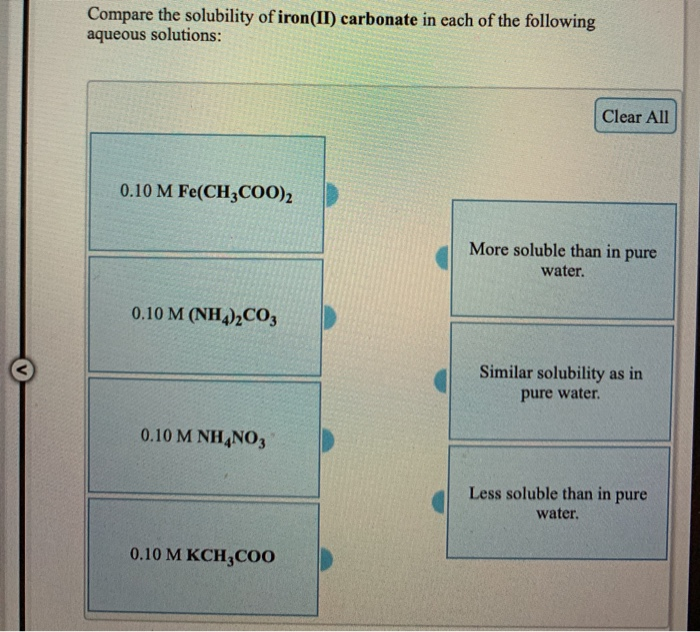
Solved Compare The Solubility Of Iron Ii Sulfide In Each O Question: compare the solubility of iron (ii) sulfide in each of the following aqueous solutions: clear all 0.10 m fe (no3)2 0.10 m (nh4)2s 0.10 m. more soluble than in pure water. similar solubility as in pure water. less soluble than in pure water. there are 2 steps to solve this one. Chemistry. chemistry questions and answers. compare the solubility of iron (ii) carbonate in each of the following aqueous solutions: clear all 0.10 m fe (ch3coo)2 more soluble than in pure water. 0.10 m (nh4)2co3 similar solubility as in pure water. 0.10 m kch3coo less soluble than in pure water. 0.10 m nh no3.

Solved Compare The Solubility Of Iron Ii Sulfide In Each Of Cheg That is, if all the solid is dissolved, you may have an unsaturated solution, and the solubility product defines the concentration of a saturated solution. at the end of this chapter is a solubility product table for ionic compounds at 25 o c. lets look at the solubility product for calcium phosphate. An overcooked hard boiled egg, showing the distinctive green coating on the yolk caused by the presence of iron (ii) sulfide. iron sulfides occur widely in nature in the form of iron–sulfur proteins . as organic matter decays under low oxygen (or hypoxic) conditions such as in swamps or dead zones of lakes and oceans, sulfate reducing. An increase in entropy (a decrease in order) favors dissolution. 13.2: types of solutions and solubility. solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. the component present in the greatest amount is the solvent, and the …. Henry's law dictates that when temperature is constant, the solubility of the gas corresponds to it's partial pressure. consider the following formula of henry's law: p = kh c (3) (3) p = k h c. where: p p is the partial pressure of the gas above the liquid, kh k h is henry's law constant, and. c c is the concentrate of the gas in the liquid.

Solved Compare The Solubility Of Iron Ii Sulfide In Each Of Cheg An increase in entropy (a decrease in order) favors dissolution. 13.2: types of solutions and solubility. solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. the component present in the greatest amount is the solvent, and the …. Henry's law dictates that when temperature is constant, the solubility of the gas corresponds to it's partial pressure. consider the following formula of henry's law: p = kh c (3) (3) p = k h c. where: p p is the partial pressure of the gas above the liquid, kh k h is henry's law constant, and. c c is the concentrate of the gas in the liquid. Therefore, iron(iii) sulfide is expected to have similar solubility in this solution as in pure water. 0.10 m kno3: this solution does not contain any ions that can interact with iron(iii) sulfide. answer therefore, iron(iii) sulfide is expected to be less soluble in this solution than in pure water. In this study, a plug flow reactor was built to measure iron sulfide solubility at various temperatures (25–90°c), ph (5.9–6.9), and ionic strength (0.150–4.27 mol kg). using the mackinawite solubility data measured in this study and in literature, a new pitzer theory based thermodynamic model with the explicit presence of ion complexes.
Solved Compare The Solubility Of Iron Ii Carbonate In Each Chegg Therefore, iron(iii) sulfide is expected to have similar solubility in this solution as in pure water. 0.10 m kno3: this solution does not contain any ions that can interact with iron(iii) sulfide. answer therefore, iron(iii) sulfide is expected to be less soluble in this solution than in pure water. In this study, a plug flow reactor was built to measure iron sulfide solubility at various temperatures (25–90°c), ph (5.9–6.9), and ionic strength (0.150–4.27 mol kg). using the mackinawite solubility data measured in this study and in literature, a new pitzer theory based thermodynamic model with the explicit presence of ion complexes.

Comments are closed.