Solved Determine Compositions Of Liquid And Solid Phases In Chegg

Solved 44 Which Of The Solid Liquid Phase Equilibria In Chegg To find the proportion of the solid phase present, use the lever rule with the given composition of nickel and the solidus and liquidus compositions at the specified temperature. determine compositions of liquid and solid phases in the cu ni system at an aggregate composition of 50% nickel and a temperature of 1260°c (2300°f): (3') liquid. Determine the liquid and solid phase compositions for a nominal composition of 30% sn and 70% pb at 250°c (482°f) your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.

Solved Ure Determine The Compositions Of The Phases Chegg Rule 1 phase composition: to determine the composition of phases which are stable at a given temperature we draw a horizontal line at the given temperature. the projections (upon the abscissa) of the intersections of the isothermal line with the liquidus and the solidus give the compositions of the liquid and solid, respectively, which. 1120 15 solid 17 liquid 07 problem #2 draw schematic phase diagrams for binary systems with (a) complete liquid and solid solubility, (b) complete liquid but zero solid solubility, and (c) complete liquid and limited solid solubility. (in your sketches label phase fields and give characteristic temperatures.) composition 1200 55 solid 55 (b). A typical phase diagram for such a mixture is shown in figure 8.6.2 8.6. 2. some combinations of substances show both an upper and lower critical temperature, forming two phase liquid systems at temperatures between these two temperatures. an example of a combination of substances that demonstrate the behavior is nicotine and water. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. in the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance. this is the phase diagram for a typical pure substance. these diagrams (including this one) are nearly.
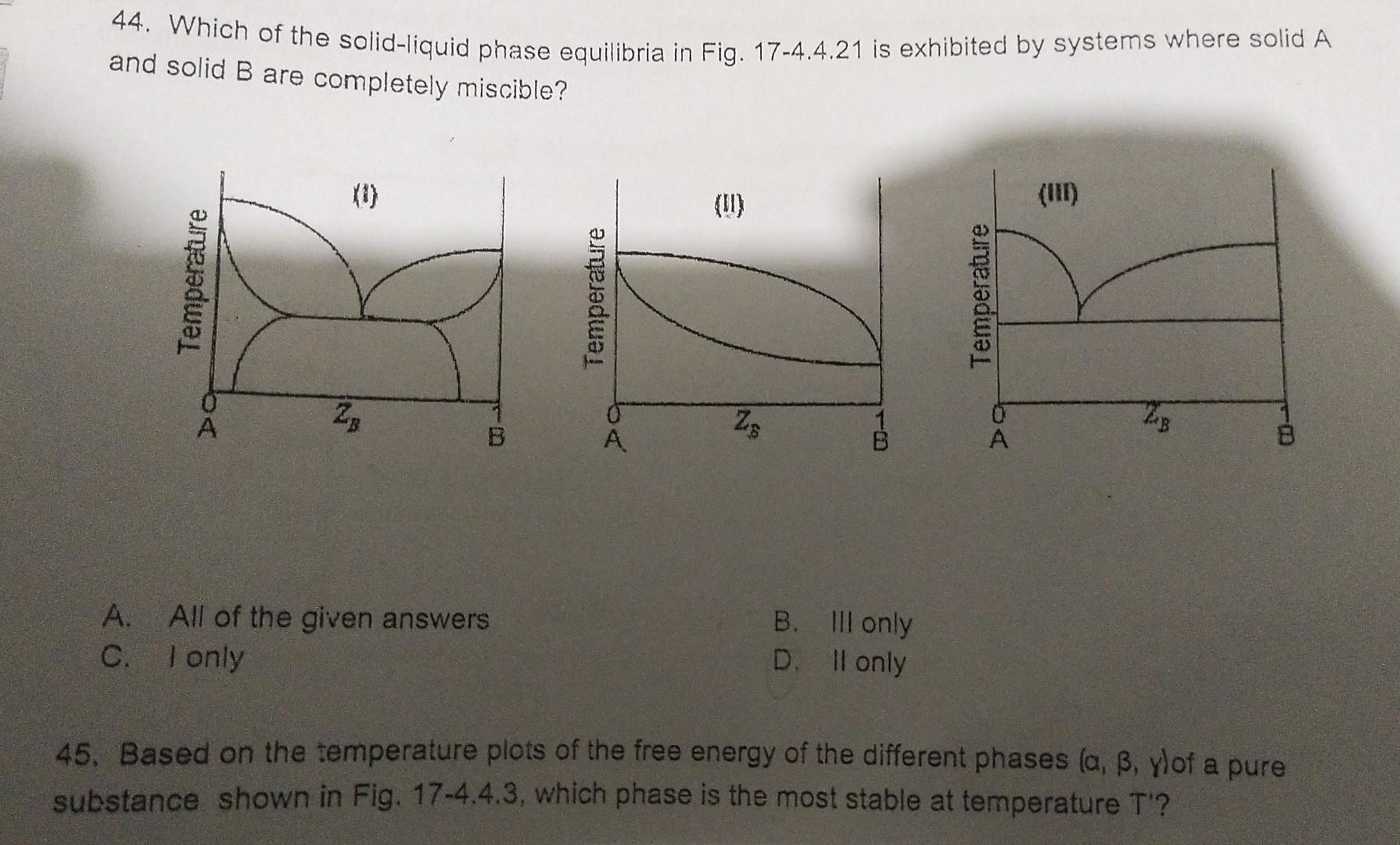
Solved 44 Which Of The Solid Liquid Phase Equilibria In Chegg A typical phase diagram for such a mixture is shown in figure 8.6.2 8.6. 2. some combinations of substances show both an upper and lower critical temperature, forming two phase liquid systems at temperatures between these two temperatures. an example of a combination of substances that demonstrate the behavior is nicotine and water. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. in the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance. this is the phase diagram for a typical pure substance. these diagrams (including this one) are nearly. 10—19 a nb—60 wt% w alloy is heated to 28000c. determine (a) the composition of the solid and liquid phases in both and and (b) the amount of each (c) assuming that the density of the solid is phase in both and . 16.05 g cm3 and that of the liquid is 13. 91 g cm3, determine the amount of each phase in vol%. (see figure 10 18) x 100% x 100%. Determine the liquid and solid phase compositions for a nominal composition of 90% sn and 10% pb at 204°c (400°f ). then use the inverse lever rule to determine the proportions of liquid and solid phases present in the alloy. there are 3 steps to solve this one.

Solved 3 Using Figure 1 Determine The Liquid And Solid Chegg 10—19 a nb—60 wt% w alloy is heated to 28000c. determine (a) the composition of the solid and liquid phases in both and and (b) the amount of each (c) assuming that the density of the solid is phase in both and . 16.05 g cm3 and that of the liquid is 13. 91 g cm3, determine the amount of each phase in vol%. (see figure 10 18) x 100% x 100%. Determine the liquid and solid phase compositions for a nominal composition of 90% sn and 10% pb at 204°c (400°f ). then use the inverse lever rule to determine the proportions of liquid and solid phases present in the alloy. there are 3 steps to solve this one.

Comments are closed.