Solved Examples 1 What Is The Percent By Mass Of A 285 G Chegg
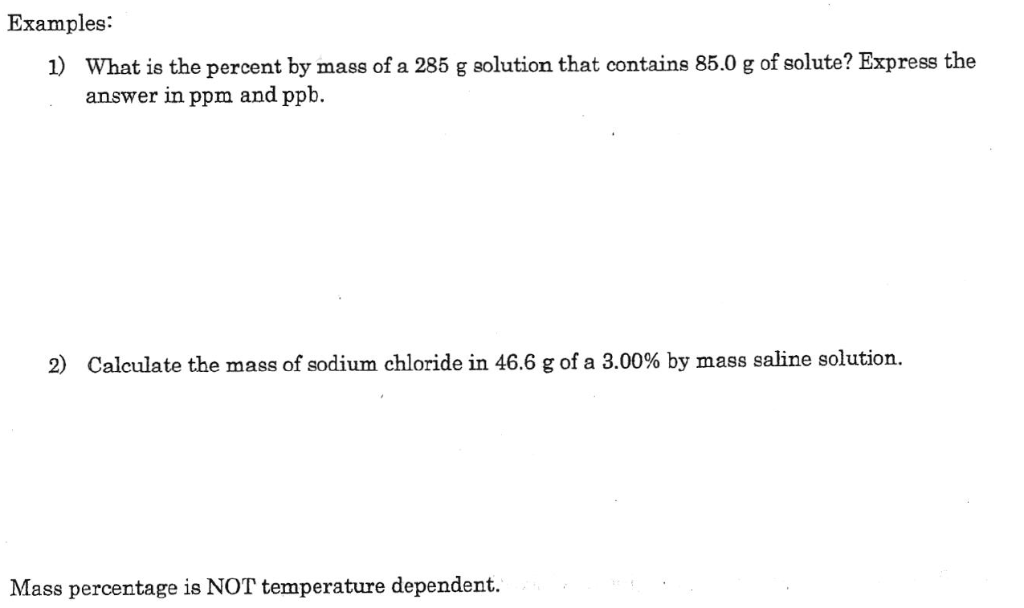
Solved Examples 1 What Is The Percent By Mass Of A 285 G Chegg Question: examples 1) what is the percent by mass of a 285 g solution that contains 85.0 g of solute? express the answer in ppm and ppb. 2) calculate the mass of sodium chloride in 46.6 g of a 3.00% by mass saline solution. mass percentage is not temperature dependent. show transcribed image text. here’s the best way to solve it. The equation is filled in, so now simply solve to calculate the mass percent. divide the mass of the chemical by the entire mass of the compound and multiply by 100. this may offer you the mass percent of the chemical. solved examples for mass percent formula. q] calculate the grams of naocl (6.15% by mass) in 285 grams of a billboard bleach.

Solved What Is The Mass Percent Of A Solution Prepared By Chegg The mass mass percent (% m m) is defined as the mass of a solute divided by the mass of a solution times 100: %m m = massofsolute massofsolution × 100%. mass of solution = mass of solute mass solvent. if you can measure the masses of the solute and the solution, determining the mass mass percent is easy. each mass must be expressed in the. Solved example on mass by mass percentage formula. q.1. a sugar syrup of mass 214.2 g contains 34.2 g of sugar. calculate the concentration of sugar in the syrup. ans: mass of solute = 34.2 g. mass of the solution = 214.2 g. ∴ mass percentage sugar in the syrup = m a s s o f s o l u t e m a s s o f s o l u t i o n × 100. The percentage concentration of any solution is most commonly expressed as mass percent: mass % of any component of the solution = (mass of the component in the solution total mass of the solution) x 100 other methods are: volume percentage: volume % of a component = (volume of the component total volume of the solution) x 100 mass by volume percentage: it is the mass of solute dissolved in. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. the formula is: mass percent = (mass of component total mass) x 100%. or. mass percent = (mass of solute mass of solution) x 100%. usually, mass is expressed in grams, but any unit of measure is acceptable as.

Solved What Is The Percent By Mass Of A Solution Made With Chegg The percentage concentration of any solution is most commonly expressed as mass percent: mass % of any component of the solution = (mass of the component in the solution total mass of the solution) x 100 other methods are: volume percentage: volume % of a component = (volume of the component total volume of the solution) x 100 mass by volume percentage: it is the mass of solute dissolved in. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. the formula is: mass percent = (mass of component total mass) x 100%. or. mass percent = (mass of solute mass of solution) x 100%. usually, mass is expressed in grams, but any unit of measure is acceptable as. Percent composition indicates the relative amounts of each element in a compound. for each element, the mass percent formula is: % mass = (mass of element in 1 mole of the compound) (molar mass of the compound) x 100%. or. mass percent = (mass of solute mass of solution) x 100%. the units of mass are typically grams. Example problems. problem 1: find the percent composition of all the elements in one mole of carbon dioxide (co 2). solution. the molar masses of carbon and oxygen are: c: 12 g. o: 16 g. molar mass of co 2 is = 12 g x 2 x 16 g = 44 g. the percent compositions are as follows: c: 12 g 44 g x 100 = 27.3%.

Solved 1 Calculate The Mass Percent Of A Solution Chegg Percent composition indicates the relative amounts of each element in a compound. for each element, the mass percent formula is: % mass = (mass of element in 1 mole of the compound) (molar mass of the compound) x 100%. or. mass percent = (mass of solute mass of solution) x 100%. the units of mass are typically grams. Example problems. problem 1: find the percent composition of all the elements in one mole of carbon dioxide (co 2). solution. the molar masses of carbon and oxygen are: c: 12 g. o: 16 g. molar mass of co 2 is = 12 g x 2 x 16 g = 44 g. the percent compositions are as follows: c: 12 g 44 g x 100 = 27.3%.

Comments are closed.