Solved Experiment 1 Concentration Determination Of Kmno4 Chegg
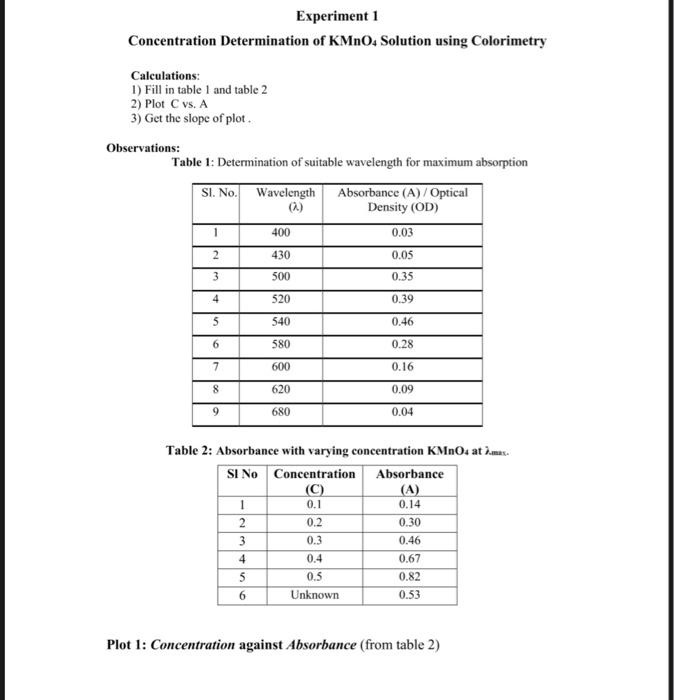
Solved Experiment 1 Concentration Determination Of Kmno4 Chegg Chemistry questions and answers. experiment 1 concentration determination of kmno4 solution using colorimetry calculations: 1) fill in table 1 and table 2 2) plot c vs. a 3) get the slope of plot. observations: table 1: determination of suitable wavelength for maximum absorption si. no. wavelength absorbance (a) optical density (od) 1 400 0. Place a cell containing 0.3 mm solution of kmno4 solution, measure the absorbance and write in table 1 • change the wavelength to 43 (equivalent to 430 nm) and repeat the last two steps • the measurement must be repeated for other wavelength by following the above steps. enter the absorbance values for all wavelength given table 1 and plot.
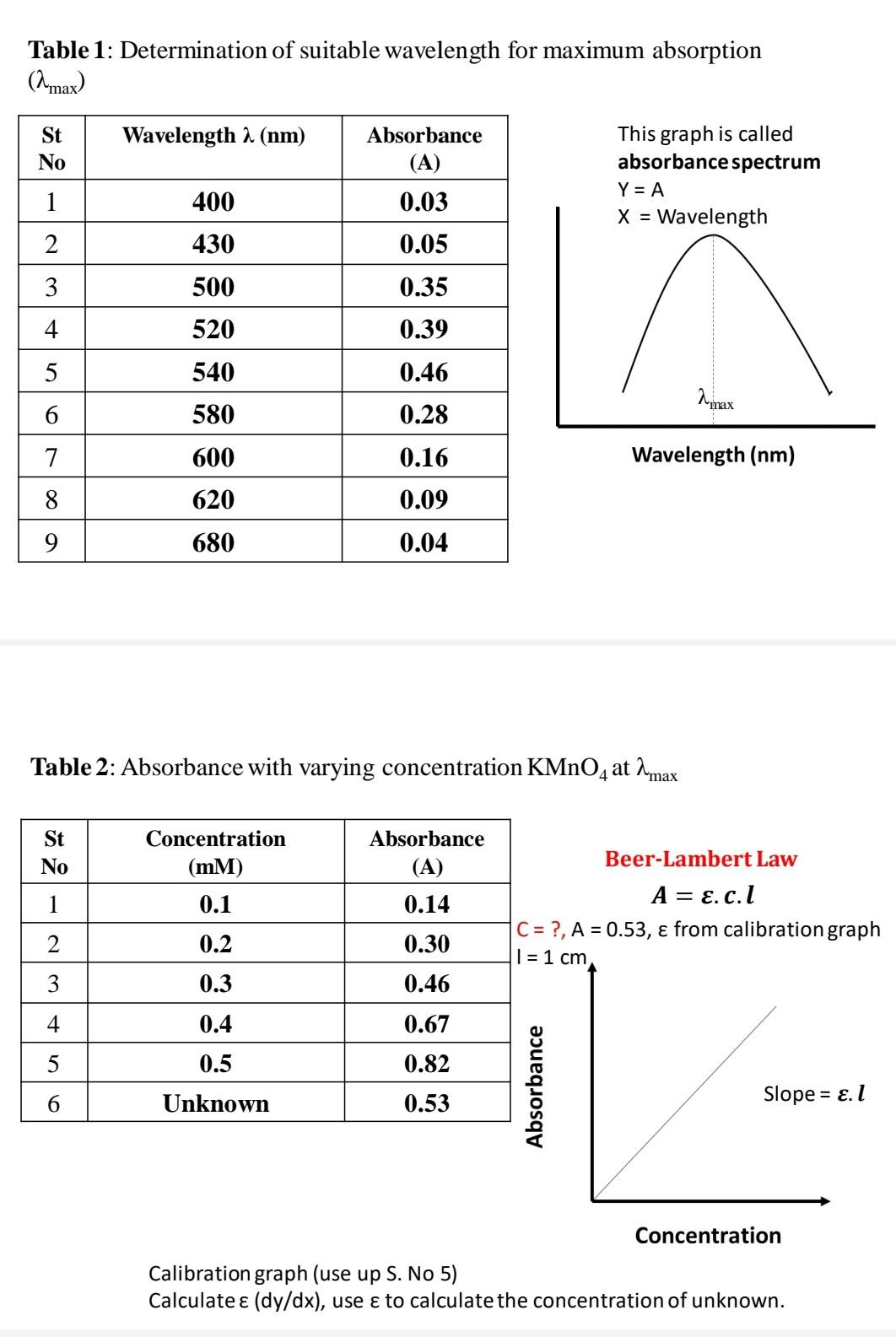
Solved Concentration Determination Of Kmno4 Solution By Chegg Here’s the best way to solve it. experiment determination of the concentration of an unknown kmno4 solution by colorimetrically heading aim of the experiment materials required & chemicals required • principal procedure calculation (tables, graphs) result. The \( \ce{kmno4} \) solution provided by the storeroom has an approximate concentration of 0.02 m. you will be given the exact concentration so that you can complete the necessary calculations. use a 50 ml beaker to get 40 ml potassium permanganate solution. transfer the solution into the burette provided. The concentration of a solution of potassium permanganate, kmno4, can be determined by titration with a known amount of oxalic acid, h2c2o4, according to the following equation: what is the concentration of a kmno4 solution if 22.35 ml reacts with 0.5170 g of oxalic acid? (lo 4.22) (a) 0.6423 m (b) 0.1028 m (c) 0.4161 m (d) 0.2569 m. M 1 (concentration of potassium permanganate) = ———— molar. v 1 (average volume of potassium permanganate) = ————ml. m 2 (concentration of oxalic acid) = 0.1 molar. v 2 (volume of oxalic acid) = 10 ml. a 1 (the number of electrons gained per formula unit of potassium permanganate in the balanced chemical equation of half cell.

Solved Experiment 1 Concentration Determination Of Kmno4 Chegg The concentration of a solution of potassium permanganate, kmno4, can be determined by titration with a known amount of oxalic acid, h2c2o4, according to the following equation: what is the concentration of a kmno4 solution if 22.35 ml reacts with 0.5170 g of oxalic acid? (lo 4.22) (a) 0.6423 m (b) 0.1028 m (c) 0.4161 m (d) 0.2569 m. M 1 (concentration of potassium permanganate) = ———— molar. v 1 (average volume of potassium permanganate) = ————ml. m 2 (concentration of oxalic acid) = 0.1 molar. v 2 (volume of oxalic acid) = 10 ml. a 1 (the number of electrons gained per formula unit of potassium permanganate in the balanced chemical equation of half cell. I then did the mcbride experiment to find $75\%$ of the volume of $\ce{kmno4}$ need to titrate the solution. i then did the experiment three times to get an average volume of $\ce{kmno4}$ to be $\pu{25.48 ml}$. and the volume of sodium oxalate solution is $\pu{25 ml}$. i should find the concentration of $\ce{kmno4}$ now. i know the molar ratio. Once the absorbance values are taken, a beer’s law plot for kmno4 is generated and the concentration of the unknown solution is determined. in this experiment have 5 parts a to e. the first part is preparation of kmno4 solutions, 0 g kmno4 are used and 5 ml, 10 ml, 15 ml and 20 ml transferred into small beaker and labelled as 5 ppm, 10 ppm.

Solved Concentration Determination Of Kmno4 Solution By Chegg I then did the mcbride experiment to find $75\%$ of the volume of $\ce{kmno4}$ need to titrate the solution. i then did the experiment three times to get an average volume of $\ce{kmno4}$ to be $\pu{25.48 ml}$. and the volume of sodium oxalate solution is $\pu{25 ml}$. i should find the concentration of $\ce{kmno4}$ now. i know the molar ratio. Once the absorbance values are taken, a beer’s law plot for kmno4 is generated and the concentration of the unknown solution is determined. in this experiment have 5 parts a to e. the first part is preparation of kmno4 solutions, 0 g kmno4 are used and 5 ml, 10 ml, 15 ml and 20 ml transferred into small beaker and labelled as 5 ppm, 10 ppm.

Solved Concentration Determination Of Kmno4 Solution By Chegg

Comments are closed.