Solved If All Sulfites And Bisulfites React With Acids To Chegg

Solved If All Sulfites And Bisulfites React With Acids To Chegg Expert verified. answer is …. if all sulfites and bisulfites react with acids to produce sulfur dioxide gas, water and a salt, which of the following best describes the net ionic equation for the reaction of aqueous solution of thydrochloric acid with solid sodium bisulfite? a. h (aq) hso3( s)→h2o(l) so2(g) b. Expert verified. displacement reactions worksheet identify the type of displacement reaction (e.g., precipitation, gas formation, or strong acid strong base) and write balanced molecular, ionic, and net ionic equations (nie) for each of the following reactions. assume all reactions occur in aqueous solution.
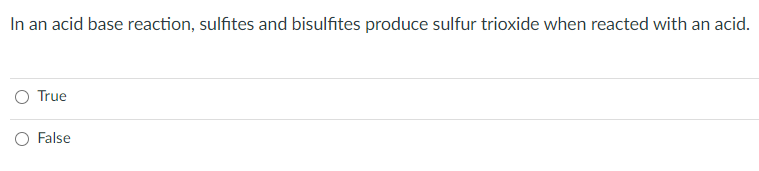
Solved In An Acid Base Reaction Sulfites And Bisulfites Chegg Question: in an acid base reaction, sulfites and bisulfites produce sulfur trioxide when reacted with an acid. true o false in a precipitation reaction, if lithium carbonate is produced, it will be a solid. Intermediate product: nh4oh. gas evoloved: nh3. gas evolution reaction. a reaction that occurs in solution and forms a gas as one of the products. study with quizlet and memorize flashcards containing terms like sulfides, cabonates bicarbonates, sulfites bisulfites, ammonium, sulfides s2, carbonates and bicarbonates co3 hco3 and more. The ionization reaction of acetic acid is as follows: ch3co2h(l)h2o (l) ⇌ h (aq) ch3co − 2 (aq) although acetic acid is very soluble in water, almost all of the acetic acid in solution exists in the form of neutral molecules (less than 1% dissociates), as we stated in section 4.1. We have an expert written solution to this problem! 5.18 if a reaction occurs in the gas phase at stp, we can determine the mass of a product from the volumes of reactants. explain. gas = 22.414l. volume of limiting reagent (l) x 1 mol limiting reagent 22.141l x c mol product a mol limiting reagent x g product 1 mol product. study with quizlet.

Solved In An Acid Base Reaction Sulfites And Bisulfites Chegg The ionization reaction of acetic acid is as follows: ch3co2h(l)h2o (l) ⇌ h (aq) ch3co − 2 (aq) although acetic acid is very soluble in water, almost all of the acetic acid in solution exists in the form of neutral molecules (less than 1% dissociates), as we stated in section 4.1. We have an expert written solution to this problem! 5.18 if a reaction occurs in the gas phase at stp, we can determine the mass of a product from the volumes of reactants. explain. gas = 22.414l. volume of limiting reagent (l) x 1 mol limiting reagent 22.141l x c mol product a mol limiting reagent x g product 1 mol product. study with quizlet. The reaction of an acid and a base is called a neutralization reaction. although acids and bases have their own unique chemistries, the acid and base "cancel" each other's chemistry to produce a rather innocuous substance—water. in fact, the general reaction between an acid and a base is: acid base → water salt acid base → water salt. A salt is a general chemical term for any ionic compound formed from an acid and a base. in reactions where the acid is a hydrogen ion containing compound and the base is a hydroxide ion containing compound, water is also a product. the general reaction is as follows: acid base → water salt. the reaction of acid and base to make water and.

Solved In An Acidic Solution Bisulfite Ion Reacts With Chegg The reaction of an acid and a base is called a neutralization reaction. although acids and bases have their own unique chemistries, the acid and base "cancel" each other's chemistry to produce a rather innocuous substance—water. in fact, the general reaction between an acid and a base is: acid base → water salt acid base → water salt. A salt is a general chemical term for any ionic compound formed from an acid and a base. in reactions where the acid is a hydrogen ion containing compound and the base is a hydroxide ion containing compound, water is also a product. the general reaction is as follows: acid base → water salt. the reaction of acid and base to make water and.

Comments are closed.