Solved The Kmno4 Solution Is First Standardized By Titration Using
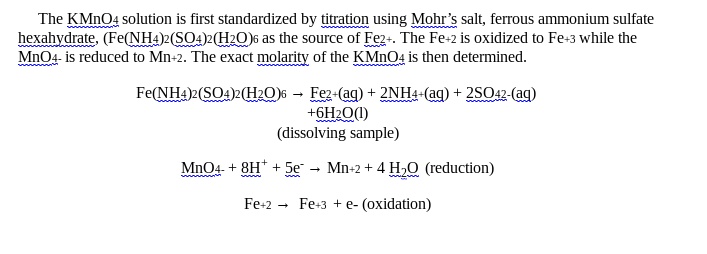
Solved The Kmno4 Solution Is First Standardized By Titration Using The moment there is an excess of potassium permanganate present the solution becomes purple. thus, kmno 4 serves as self indicator in acidic solution. potassium permanganate is standardized against pure oxalic acid. it involves a redox reaction. oxalic acid is oxidised to carbon dioxide by kmno 4, which itself gets reduced to mnso 4. oxalic. Step 2: titration of kmno4 against standard oxalic acid solution. at first, fill the burette with kmno 4 solution. then take out 10ml of 0.1m standard oxalic acid solution into a conical flask. add a few drops of h 2 so 4 in order to prevent oxidation of manganese to form manganese dioxide.
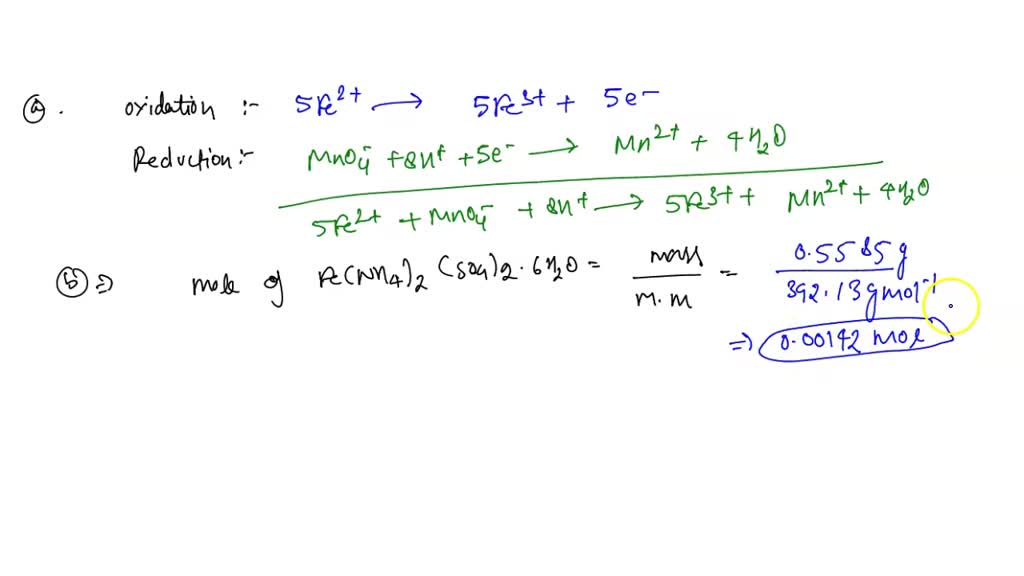
Solved The Kmno4 Solution Is First Standardized By Titration Using Standard deviation =. standardization of 0.02 m kmno4. dry ~ 1.5 g of pure na2c2o4 at 110 ºc for ~ 30 minutes. weigh (to ± 0.1 mg) three 0.25 g samples of na2c2o4. transfer the samples quantitatively to three 400 ml beakers. dissolve each sample in ~ 200 ml of 1 m h2so4. heat each solution to 80 90 ºc and titrate with kmno4 while stirring. A student was titrating 25.00 ml of a basic solution with an hcl solution that was 0.281 m. the student ran out of the hcl solution after having added 32.46 ml, so she borrowed an hcl solution that was labeled as 0.317 m. an additional 11.5 ml of the second solution was needed to complete the titration. Therefore, when a solution of a reducing agent is added to a permanganate solution, the solution is decolourised. the moment there is an excess of potassium permanganate present, the solution becomes purple. hence, kmno 4 serves as a self indicator in an acidic solution. potassium permanganate is standardized against pure oxalic acid. The standardized naoh solution can then be used to titrate a solution of an acid whose concentration is unknown. figure 4.6.2: the reaction of khp with naoh. as with all acid base reactions, a salt is formed. the topic solution stoichiometry deals with quantities in chemical reactions taking place in solutions.
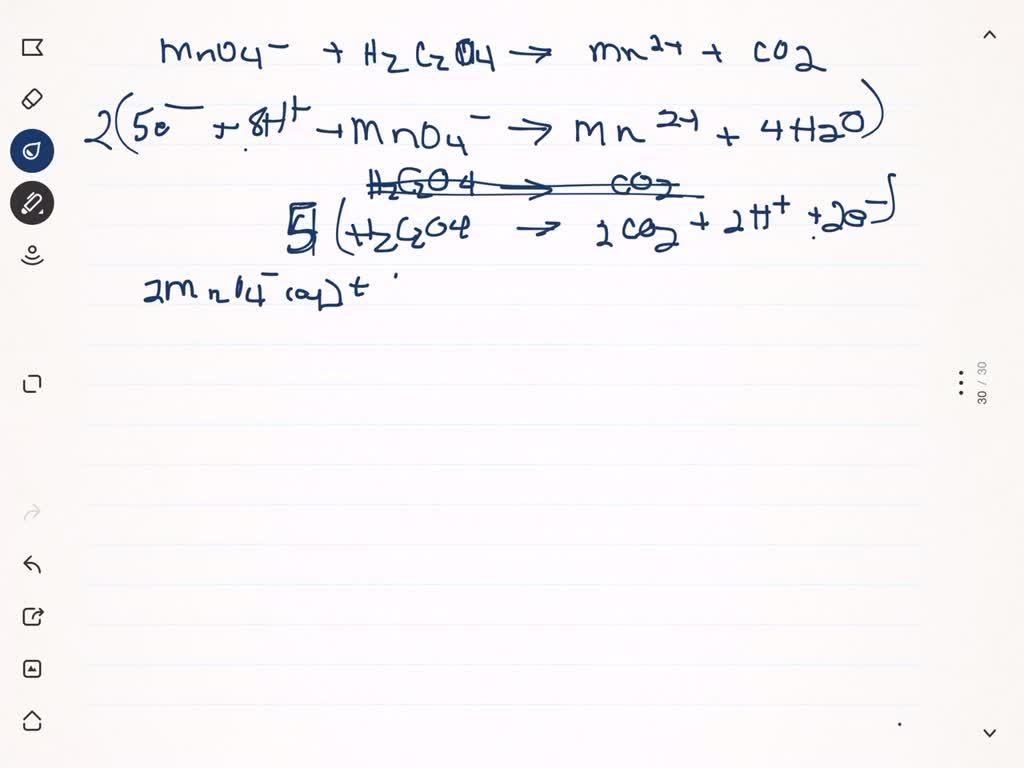
Solved For The Titration Of A Kmno4 Solution Na2c204 Is Titrated As A Therefore, when a solution of a reducing agent is added to a permanganate solution, the solution is decolourised. the moment there is an excess of potassium permanganate present, the solution becomes purple. hence, kmno 4 serves as a self indicator in an acidic solution. potassium permanganate is standardized against pure oxalic acid. The standardized naoh solution can then be used to titrate a solution of an acid whose concentration is unknown. figure 4.6.2: the reaction of khp with naoh. as with all acid base reactions, a salt is formed. the topic solution stoichiometry deals with quantities in chemical reactions taking place in solutions. A kmno 4 (aq) solution is to be standardized by titration against as 2 o 3 (s). a 0.235 g sample of as 2 o 3 requires 32.15 ml of the kmno 4 (aq) for its titration. 5 as 2 o 3 (aq) 4 mno 4 (aq) 9 h 2 o (l) 12 h (aq) > 10 h 3 aso 4 (aq) 4 mn 2 (aq) prove that this reaction is a redox reaction. show your work or thought process. A kmno4 (aq) solution is to be standardized by titration against as2o3 (s). a 0.235 g sample of as2o3 requires 32.15 ml of the kmno4 (aq) for its titration. 5 as2o3 (aq) 4 mno4 (aq) 9h20 1) 12 h (aq) > 10 h aso4 (aq) 4 mn2 (aq) prove that this reaction is a redox reaction. show your work or thought process. 1.

Comments are closed.