Standard Curve Error Bars ⇔ C 💻 Beers Law Linear Regression 3

Calibration Plot Error Bars Finding C Linear Regression Youtube Like 👍🏻 share 👆🏼 subscribe 🔔using excel to make a standard curve a.k.a. calibration plot from measurements of absorbance (a *not* abs) at variou. How to get a standard curve, a.k.a. calibration plot, in excel. data from measurements of absorbance (a) at various concentrations (c) for an organic dye, al.

Error Bars Linear Regression And Standard Deviation For Point The calibration equation is. sstd = 122.98 × cstd 0.2. figure 5.4.7 shows the calibration curve for the weighted regression and the calibration curve for the unweighted regression in example 5.4.1. although the two calibration curves are very similar, there are slight differences in the slope and in the y intercept. Line segment ab or on the linear regression displayed in figure 1. 0.0 0.2 0.4 0.6 0.8 1.0 1.2 0 500 1000 1500 2000 concentration (µg ml) absorbance 619 667 figure 2. example standard curve involving six points. the thin line is a point to point graph through the plotted standards. the thick line is a 3 parameter regression for the entire set. This relationship is known as beer’s law and is given by the equation: a= εbc (equation 4) where a is the absorbance of the solution, ε is the molar absorptivity of the solute, b is the path length of light passing through the sample of solution, and c is the concentration of the solution in molarity (moles l). Limitations to beer's law. beer’s law suggests that a plot of absorbance vs. concentration—we will call this a beer’s law plot—is a straight line with a y intercept of zero and a slope of ab or \(\varepsilon b\). in some cases a beer’s law plot deviates from this ideal behavior (see figure 3.2.9 ), and such deviations from linearity.
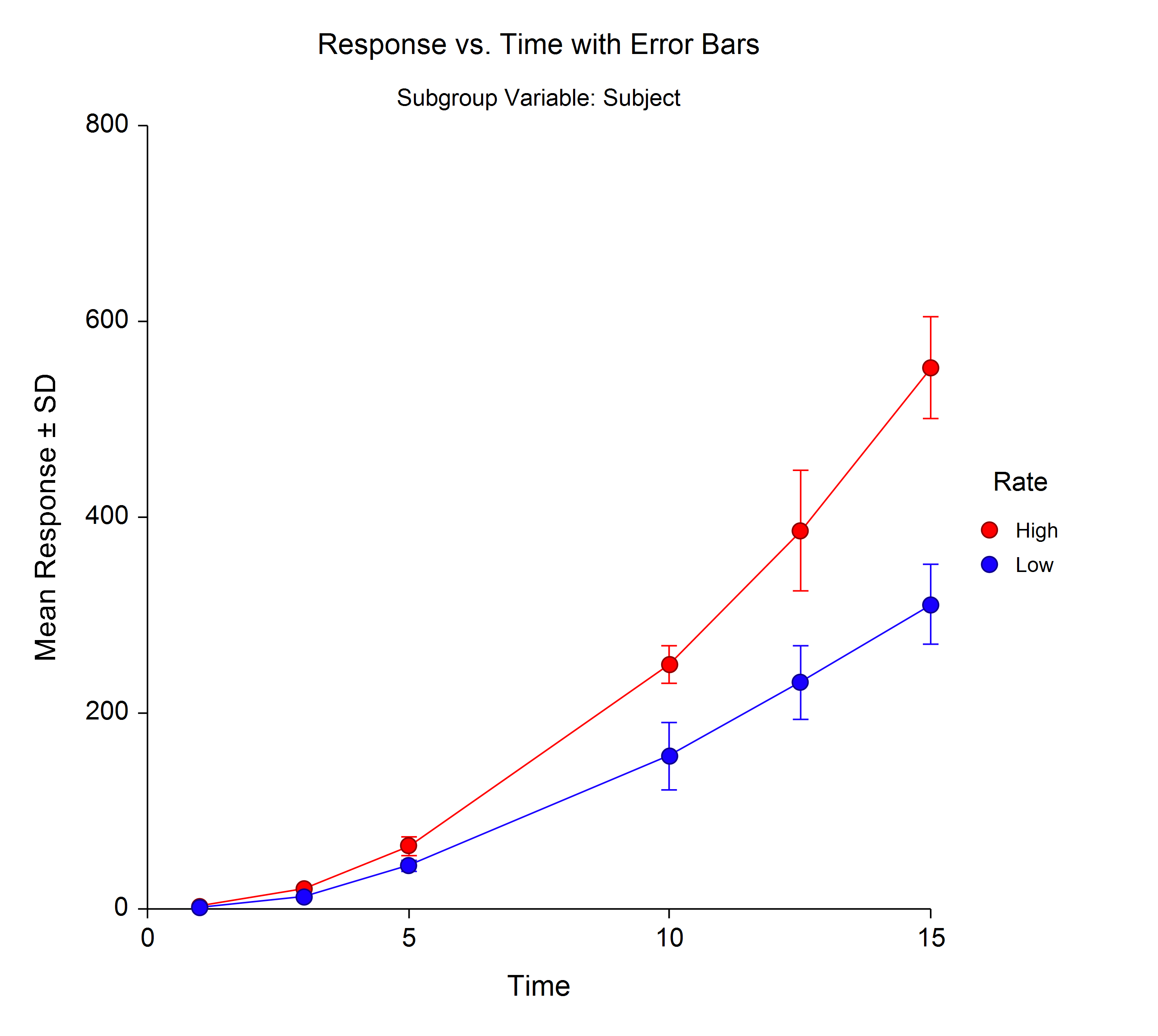
Scatter Plot With Error Bars This relationship is known as beer’s law and is given by the equation: a= εbc (equation 4) where a is the absorbance of the solution, ε is the molar absorptivity of the solute, b is the path length of light passing through the sample of solution, and c is the concentration of the solution in molarity (moles l). Limitations to beer's law. beer’s law suggests that a plot of absorbance vs. concentration—we will call this a beer’s law plot—is a straight line with a y intercept of zero and a slope of ab or \(\varepsilon b\). in some cases a beer’s law plot deviates from this ideal behavior (see figure 3.2.9 ), and such deviations from linearity. Page 3 of 9 the equation for beer’s law is a straight line with the general form of y = mx b. beer’s law: a = ( l)c with the general form y = (m)x where the slope, m, is equal to l. in this case, use the absorbance found for your unknown, along with the slope of your best fit line, to determine c, the concentration of the unknown solution. Input the path length, i.e., 1 cm. 1 \text { cm} 1 cm. the calculator will display the absorbance: 0.3637. 0.3637 0.3637. you can also use this beer lambert law calculator as a transmittance to absorbance calculator. just enter the transmittance values in percentage, and you will get the absorbance.

Curves Error Bars Techgraphonline Page 3 of 9 the equation for beer’s law is a straight line with the general form of y = mx b. beer’s law: a = ( l)c with the general form y = (m)x where the slope, m, is equal to l. in this case, use the absorbance found for your unknown, along with the slope of your best fit line, to determine c, the concentration of the unknown solution. Input the path length, i.e., 1 cm. 1 \text { cm} 1 cm. the calculator will display the absorbance: 0.3637. 0.3637 0.3637. you can also use this beer lambert law calculator as a transmittance to absorbance calculator. just enter the transmittance values in percentage, and you will get the absorbance.

Standard Error Bar Graph

Comments are closed.