Standard Reduction Potentials Table
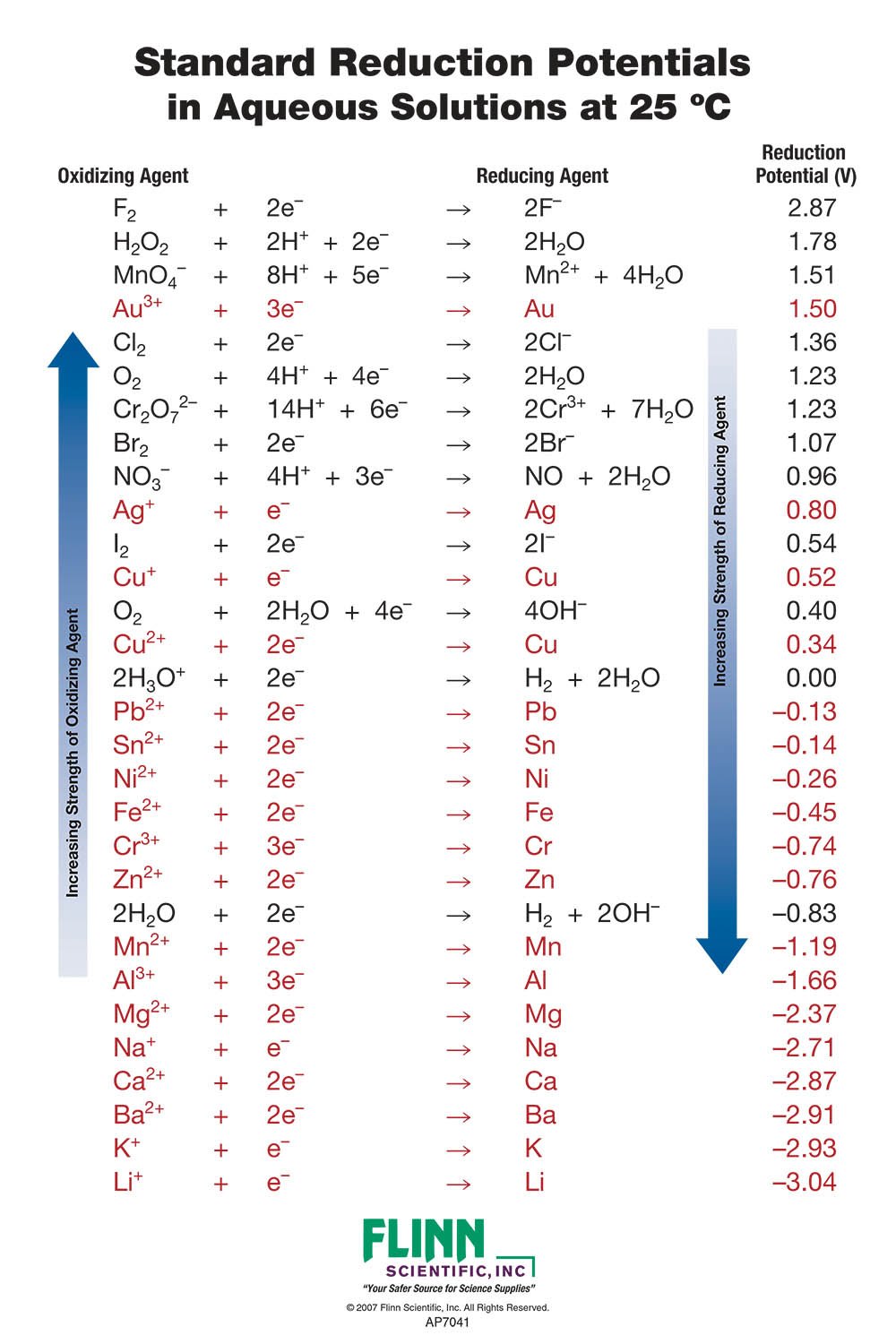
Standard Reduction Potential Charts For Chemistry The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. standard cathode (reduction) half reaction standard reduction potential e° (volts). Standard electrode potential (data page).

Extra Credit 24 Chemistry Libretexts Using table 17.3.1, the reactions involved in the galvanic cell, both written as reductions, are. au3 (aq) 3e − au(s) e ∘ au3 au = 1.498v. ni2 (aq) 2e − ni(s) e ∘ ni2 ni = − 0.257v. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. the reaction at the anode will be the half. Solids, gases, and liquids are identified; all other species are aqueous. reduction reactions in acidic solution are written using h in place of h 3 o . you may rewrite a reaction by replacing h with h 3 o and adding to the opposite side of the reaction one molecule of h 2 o per h ; thus. h3aso4 2h 2e– ↽−−⇀ haso2 2h2o h. Table of standard reduction potentials (modified from ) the values of standard electrode potentials are given in the table in . volts. relative to the . Standard reduction potentials table.

Unit 7 Nuclear And Kinetics Teaching Chemistry Chemistry Lessons Table of standard reduction potentials (modified from ) the values of standard electrode potentials are given in the table in . volts. relative to the . Standard reduction potentials table. Standard electrode potentials in aqueous solution at 25°c cathode (reduction) half reaction: standard potential e tables reference. Learn how to use standard reduction potentials to determine standard cell potentials and compare oxidizing and reducing agents. see examples of galvanic cells and calculations using the standard hydrogen electrode as a reference.
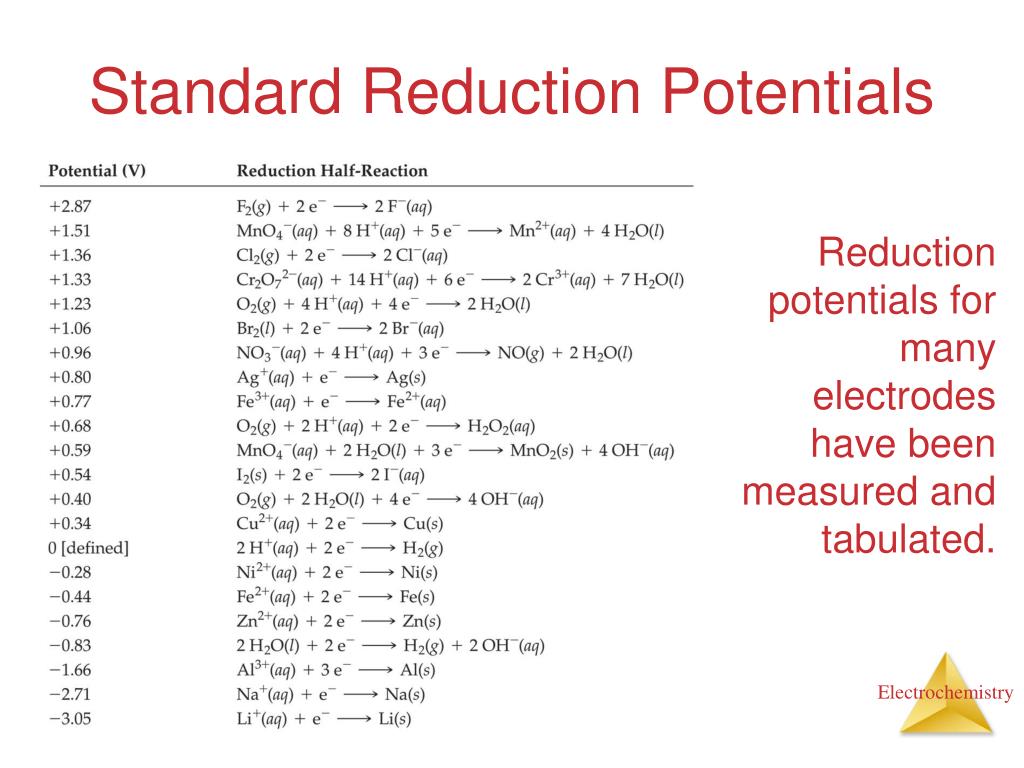
Ppt Chapter 20 Electrochemistry Powerpoint Presentation Free Standard electrode potentials in aqueous solution at 25°c cathode (reduction) half reaction: standard potential e tables reference. Learn how to use standard reduction potentials to determine standard cell potentials and compare oxidizing and reducing agents. see examples of galvanic cells and calculations using the standard hydrogen electrode as a reference.

Comments are closed.