Standarization Of Kmno4 Docx Aim To Standardize Kmno4 Using 0 05 0

Standarization Of Kmno4 Docx Aim To Standardize Kmno4 Usingођ Potassium permanganate solution. standard solution of oxalic acid (0.1 m). sulfuric acid. procedure ( how to standardize potassium permanganate) 1.clean the burette with distilled water, then empty the water, then rinse the burette with potassium permanganate solution, and then empty the burette. And in this case, there will be permanent color change in the solution, white turns into faint pink. this faint pink color is due to the presence of very little amount of extra kmno4 as there is no more standard solution left to oxidize it. thus, kmno4 indicates that the standard solution was completely oxidized. thus, kmno4 acts as self indicator.
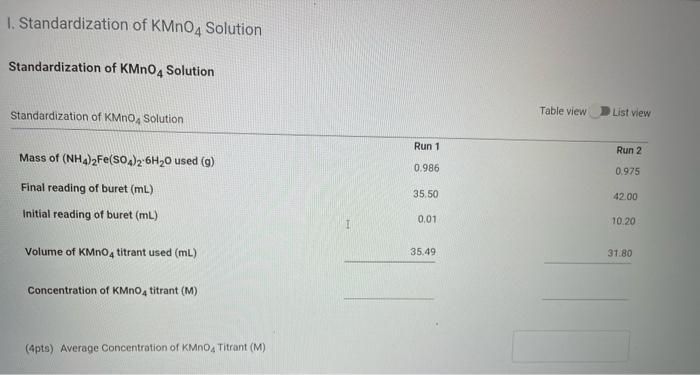
Solved 1 Standardization Of Kmno4 Solution Standardization Chegg The solution is now a 1.0 m potassium permanganate solution. standardization of 1.0 m potassium permanganate solution to prepare 1.0 n solution: to standardize the 1.0 m kmno4 solution and prepare a 1.0 n solution, you’ll need to determine its exact molar concentration. materials required: – a primary standard reagent, such as oxalic acid. Aim: to standardize kmno 4 using 0.05 0.01 m standard solution of mohr’s salt (ferrous ammounium sulphate). theory: the reaction between kmno 4 and mohr’s salt is a redox reaction and the titration is therefore called a redox titration. mohr’s salt acts as the reducing agent and kmno 4 is the oxidizing agent. Prepration and standarization of 0.1n kmno4 solution aim: to determine the concentration normality of potassium permanganate (kmno 4) solution by titrating is against a 0.1n standard solution of oxalic acid (h 2 c 2 o 4) . titration of potassium permanganate with oxalic acid is a type of redox titration . kmno. Dissolve g of potassium permanganate in 1000 ml of water, heat on a water bath for 1hour, allow to stand for 2 days, and filter through glass wool. time needed: 30 minutes. standardization of 0.02 m potassium permanganate solution. addition of potassium iodide. to 25.0 ml of the solution in a glass stoppered flask add 2 g of potassium iodide.

Standardization Of Kmno4 By Sodium Oxalate As A Primary Standard Prepration and standarization of 0.1n kmno4 solution aim: to determine the concentration normality of potassium permanganate (kmno 4) solution by titrating is against a 0.1n standard solution of oxalic acid (h 2 c 2 o 4) . titration of potassium permanganate with oxalic acid is a type of redox titration . kmno. Dissolve g of potassium permanganate in 1000 ml of water, heat on a water bath for 1hour, allow to stand for 2 days, and filter through glass wool. time needed: 30 minutes. standardization of 0.02 m potassium permanganate solution. addition of potassium iodide. to 25.0 ml of the solution in a glass stoppered flask add 2 g of potassium iodide. Potassium permanganate solution standardization. to 25.0 ml of the solution in a glass stoppered flask add 2 g of potassium iodide, followed by 10 ml of 1 m sulphuric acid. titrate the liberated iodine with 0.1 m sodium thiosulphate, using 3 ml of starch solution, added towards the end of the titration, as an indicator. On the other hand, the kmno4 solution was standardized by adding 50 ml of 3 m h2so4 and 0.05000 0.08000 g na2c2o4, which was previously dried for an hour, into a 250 ml beaker. the solution was heated to 60oc and titrated slowly with kmno4 solution, allowing 30 seconds for each drop to lose color.

Comments are closed.