Sulfur Periodic Table And Atomic Properties
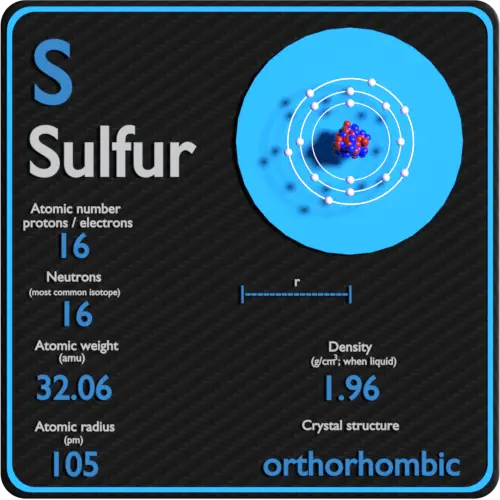
Sulfur Periodic Table And Atomic Properties Sulfur. 16. 32.06. glossary. groupa vertical column in the periodic table. members of a group typically have similar properties and electron configurations in their outer shell. perioda horizontal row in the periodic table. the atomic number of each element increases by one, reading from left to right. Sulfur – periodic table – atomic properties. sulfur is abundant, multivalent, and nonmetallic. under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula s8. elemental sulfur is a bright yellow crystalline solid at room temperature. chemically, sulfur reacts with all elements except for gold, platinum.
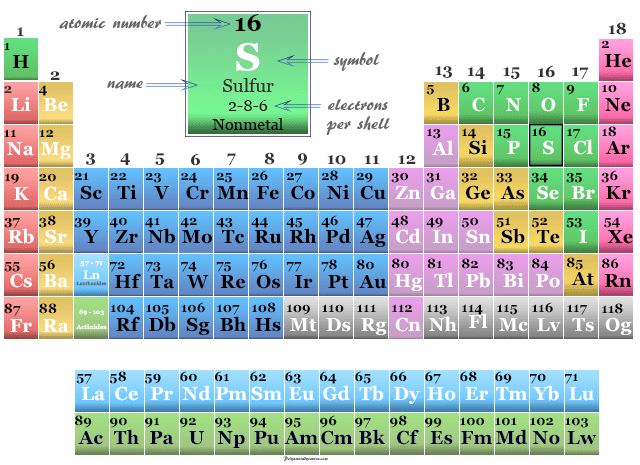
Sulfur Element Facts Properties Production Uses Sulfur is an essential component of all living cells. it is the eighth most abundant element in the human body by weight, [ 100 ] about equal in abundance to potassium, and slightly greater than sodium and chlorine. [ 101 ] a 70 kg (150 lb) human body contains about 140 grams (4.9 oz) of sulfur. [ 102 ]. Sulfur (s), nonmetallic chemical element belonging to the oxygen group (group 16 [via] of the periodic table), one of the most reactive of the elements. pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. it reacts with all metals except gold and platinum. Sulfur (s) sulfur is the 16th element in the periodic table and has a symbol of s and atomic number of 16. it has an atomic weight of 32.060 and a mass number of 32. sulfur has sixteen protons and sixteen neutrons in its nucleus, and sixteen electrons in three shells. it is located in group sixteen, period three and block p of the periodic table. Sulfur is a component of black gunpowder, and is used in the vulcanization of natural rubber and a fungicide. it is used to make sulfite paper and other papers, to fumigate, and to bleach dried fruits. it is also used extensively in making phosphatic fertilizers. elemental sulfur is considered to be of low toxicity. isotopes.

Comments are closed.