Synthesis Of Peptides Master Organic Chemistry
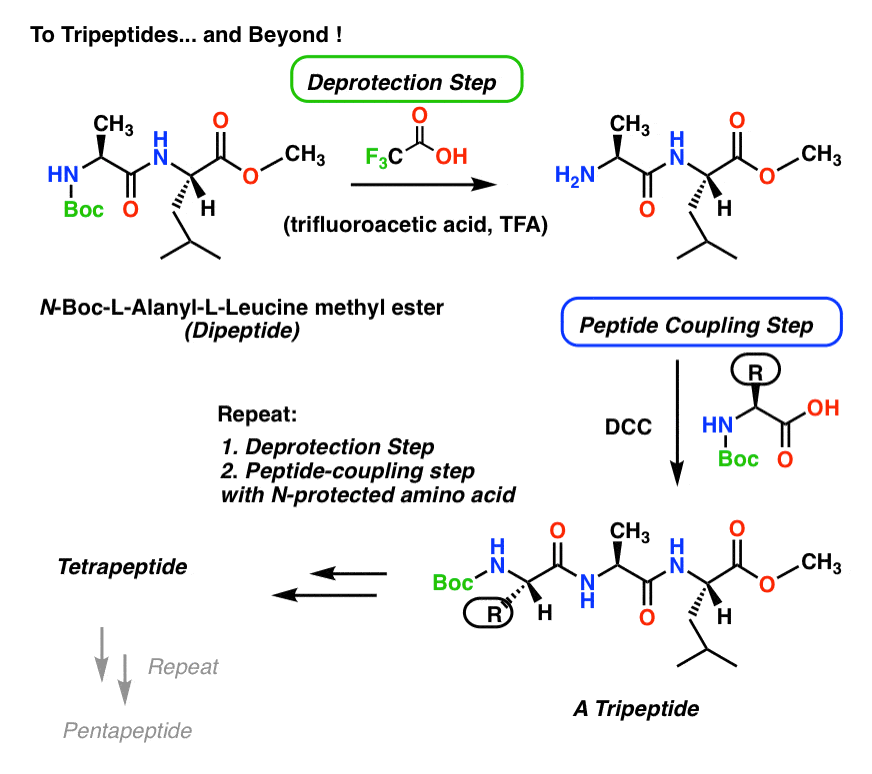
Synthesis Of Peptides вђ Master Organic Chemistry The journal of organic chemistry 1972 37 (22), 3404 3409 doi: 10.1021 jo00795a005 the discovery and development of the fmoc (9 fluorenylmethoxycarbonyl) group as a protecting group for amines has also improved the practice of peptide synthesis and spps. a mild procedure for solid phase peptide synthesis: use of fluorenylmethoxycarbonylamino acids. Once the structure of a peptide is known, its synthesis can be undertaken—perhaps to obtain a larger amount for biological evaluation. a simple amide might be formed by treating an amine and a carboxylic acid with a carbodiimide (either dcc or edc; section 21.3 ), but peptide synthesis is a more difficult problem because many different amide bonds must be formed in a specific order, rather.
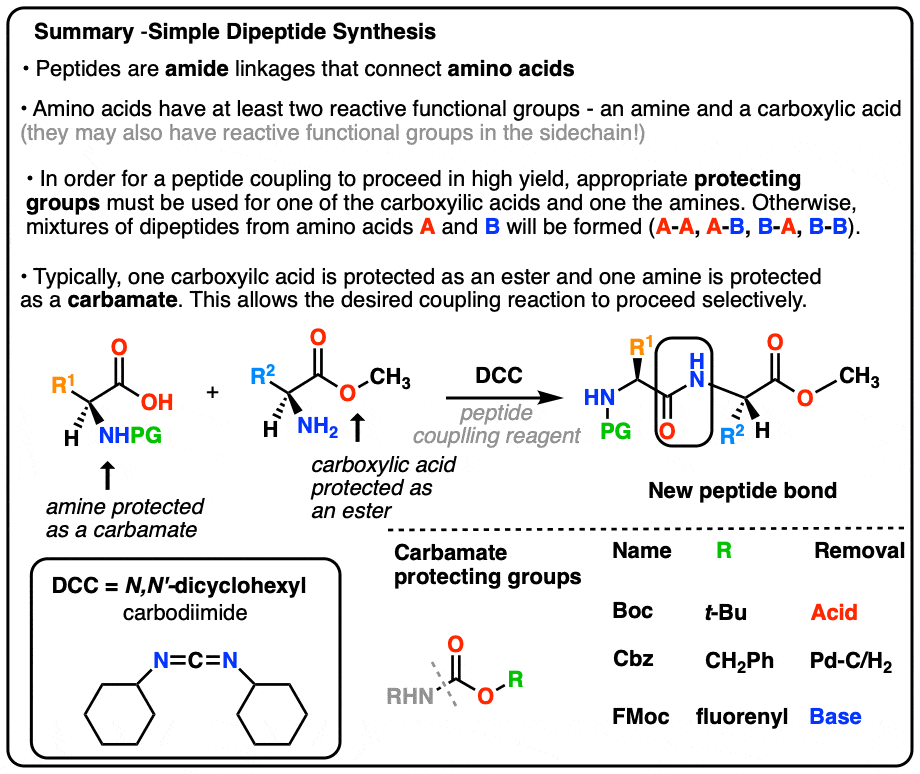
Synthesis Of Peptides вђ Master Organic Chemistry The journal of organic chemistry 1972 37 (22), 3404 3409 doi: 10.1021 jo00795a005 the discovery and development of the fmoc protecting group for amines adds another layer orthogonality to amine protection deprotection strategies. the fmoc group is base labile, and in peptide synthesis is typically removed with 20% piperidine in dmf. Once the structure of a peptide is known, its synthesis can be undertaken—perhaps to obtain a larger amount for biological evaluation. a simple amide might be formed by treating an amine and a carboxylic acid with a carbodiimide (either dcc or edc; section 21.3), but peptide synthesis is a more difficult problem because many different amide bonds must be formed in a specific order, rather. The strecker synthesis is a two step procedure for the synthesis of amino acids. it begins with the addition of cyanide ion to an imine, forming an alpha amino nitrile. this is then hydrolyzed (e.g. with strong acid) to give an alpha amino acid. by varying the r group on the imine, a wide variety of amino acids may be made this way. Outline the five steps required in order to form a dipeptide from two given amino acids. in order to synthesize a peptide from its component amino acids, two obstacles must be overcome. the first of these is statistical in nature, and is illustrated by considering the dipeptide ala gly as a proposed target.

Synthesis Of Peptides вђ Master Organic Chemistry The strecker synthesis is a two step procedure for the synthesis of amino acids. it begins with the addition of cyanide ion to an imine, forming an alpha amino nitrile. this is then hydrolyzed (e.g. with strong acid) to give an alpha amino acid. by varying the r group on the imine, a wide variety of amino acids may be made this way. Outline the five steps required in order to form a dipeptide from two given amino acids. in order to synthesize a peptide from its component amino acids, two obstacles must be overcome. the first of these is statistical in nature, and is illustrated by considering the dipeptide ala gly as a proposed target. In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. protecting group strategies are usually. In 1991 the excellent the chemical synthesis of peptides by john jones (oxford university press, 1991) appeared. it covers, in part, the same field, but is different enough from peptide chemistry, to justify publication of a revised edition of the latter.

Comments are closed.