The 4 Phases Of Clinical Trials Market Business News

Cancer Treatment Clinical Trials Doctorvisit The 4 phases of clinical trials. by marie singer published feb 11, 2022 at 22:35 pm gmt. before a sponsor may submit its therapies to the fda for consideration to be put on the market, they must complete four phases of the clinical trial procedure. the clinical trials are typically carried out by professionals such as clinical ink. The is to conduct ongoing safety surveillance, identify rare adverse reactions or harmful effects, assess efficacy, and optimise the drug's use. phase 4 clinical trials also play a big role in responding to regulatory requirements (if necessary) and supporting label expansions or new indications that might come to light.
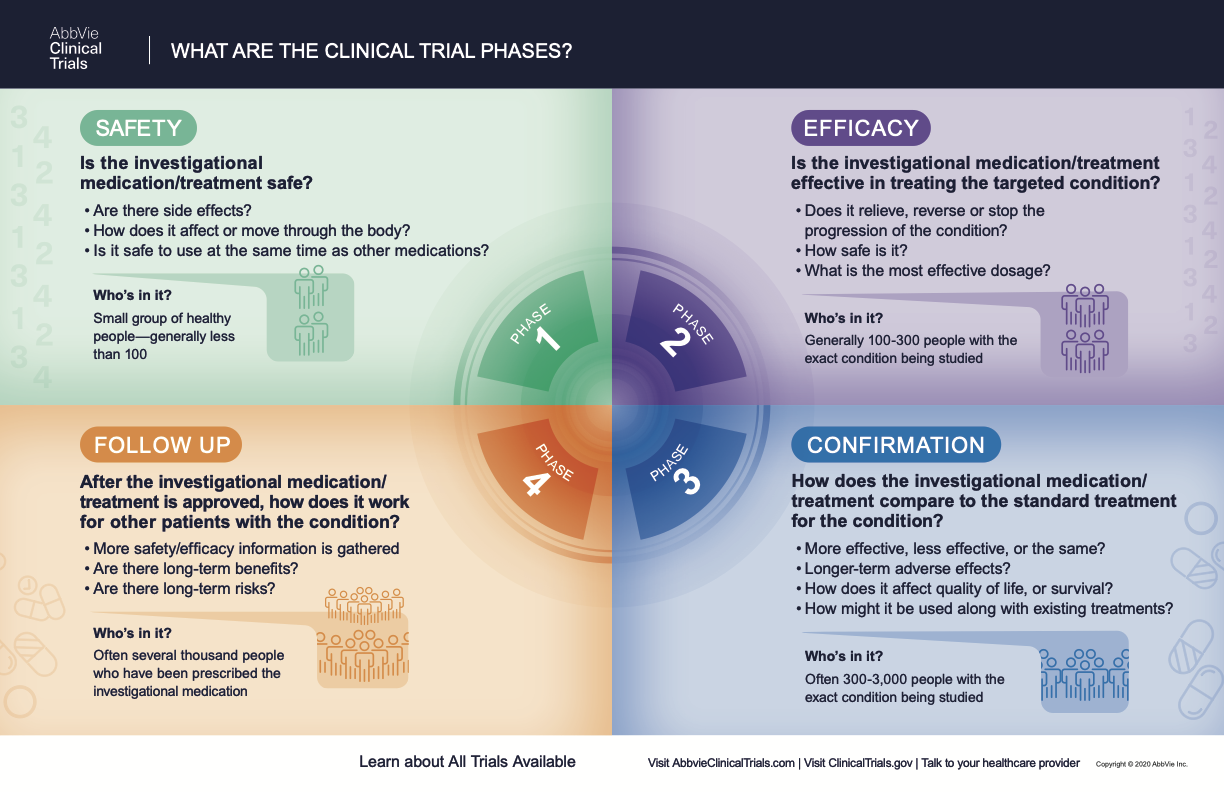
What Are The Phases Of Clinical Trials Clinical Trial Phases The clinical trials market size is estimated at usd 50.66 billion in 2024, and is expected to reach usd 67.5 billion by 2029, growing at a cagr of 5.91% during the forecast period (2024 2029). the covid 19 pandemic tremendously impacted the market for clinical trials, as there has been a rising focus on developing new therapeutics or vaccines. Phase 4. the final clinical trial stage is phase 4, or the “post marketing surveillance” phase. these trials enroll the most individuals, usually several thousand. phase 4 trials take place after the drug is already marketed and available to the general public. once again, the main objective of the trial is to further test for safety and. The phases of clinical trials. clinical trials typically have four phases. there are three phases of experimentation and one post marketing phase of regulatory oversight. it’s important to note that there are also some important steps that need to happen before a therapy even gets to humans for clinical trials. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before.

All About Cancer Clinical Trials Trial Safety Measures Masonic The phases of clinical trials. clinical trials typically have four phases. there are three phases of experimentation and one post marketing phase of regulatory oversight. it’s important to note that there are also some important steps that need to happen before a therapy even gets to humans for clinical trials. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Phase ii. phase ii clinical trials begin after a drug is determined to be safe for human use in a phase i trial. phase ii studies can involve hundreds of patients who all have the same condition—be it cancer, asthma, diabetes, or something else—that the drug is aimed to treat, and can last from a few months to a couple of years. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and.

Comments are closed.