The New Covid 19 Bivalent Vaccine Should I Get It
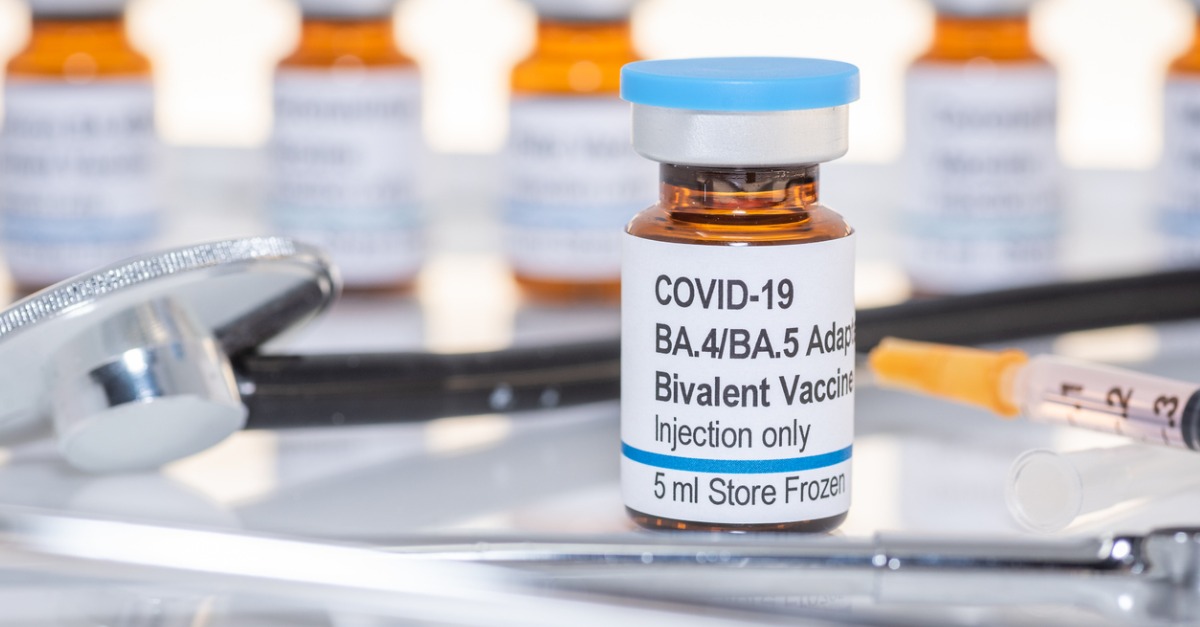
What Is The New Bivalent Covid 19 Booster And Who Should Get It Yes, because the bivalent booster will give you a better response to the currently circulating variants. the cdc recommendation is that everyone 5 years and older get the bivalent booster at least two months after their last dose or at least three months after a covid 19 infection. there is also some data that suggest waiting as long as six. The approval of spikevax (covid 19 vaccine, mrna) (2024 2025 formula) was granted to modernatx inc. and the eua amendment for the moderna covid 19 vaccine (2024 2025 formula) was issued to.
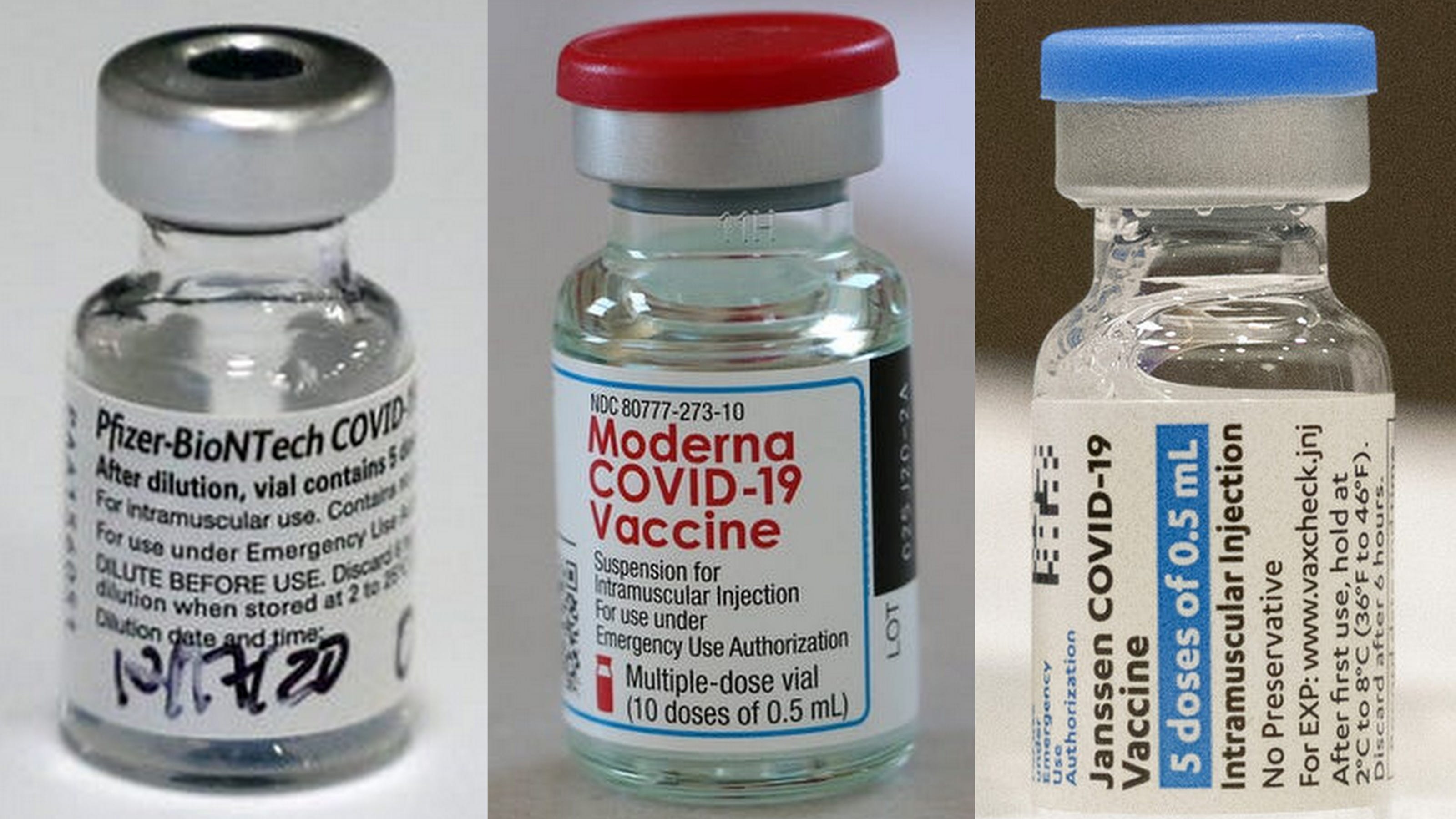
Three Covid 19 Vaccines Compared Pfizer Moderna Johnson Johnson An additional bivalent booster offers extra protection for those at high risk of covid 19 related hospitalization and death. [originally published: april 21, 2023. updated: may 17, 2023.] note: the johnson & johnson (janssen) covid 19 vaccine is no longer available in the u.s. in may 2023, existing doses of the j&j vaccine expired and the. The shots — also known as bivalent vaccines —are designed to target both the original coronavirus strain and the currently circulating omicron subvariants ba.4 and ba.5. As of may 6, 2023, covid 19–associated hospitalization rates were highest among adults aged ≥65 years (9.5 per 100,000 persons). ¶ bivalent booster doses are shown to provide the highest protection against hospitalization among adults, with protection sustained through at least 179 days against critical outcomes, including intensive care unit admission or in hospital death (4). When vaccines for covid 19 rolled out in late 2020, people of all ages rushed to get in line. we flaunted our vaccination cards on social media and helped find appointments for friends and family. three years later, only a small fraction of the eligible u.s. population has gotten the bivalent booster designed to protect against the omicron.

Pfizer Biontech Covid 19 Vaccine Booster Dose Authorized For High Risk As of may 6, 2023, covid 19–associated hospitalization rates were highest among adults aged ≥65 years (9.5 per 100,000 persons). ¶ bivalent booster doses are shown to provide the highest protection against hospitalization among adults, with protection sustained through at least 179 days against critical outcomes, including intensive care unit admission or in hospital death (4). When vaccines for covid 19 rolled out in late 2020, people of all ages rushed to get in line. we flaunted our vaccination cards on social media and helped find appointments for friends and family. three years later, only a small fraction of the eligible u.s. population has gotten the bivalent booster designed to protect against the omicron. Fda authorized for emergency use novavax covid 19 vaccine (2024 2025 formula) to more closely target currently circulating variants to provide better protection against serious consequences of. October 12, 2022. the centers for disease control and prevention (cdc) encourages those eligible to receive the new bivalent covid 19 booster vaccine. "the bivalent booster vaccine is a new vaccine that provides protection against the original strain of covid 19, as well as the two more recent variants ba.4 and ba.5," says dr. nipunie rajapakse.
Who Lists Moderna Covid 19 Vaccine For Emergency Use Fda authorized for emergency use novavax covid 19 vaccine (2024 2025 formula) to more closely target currently circulating variants to provide better protection against serious consequences of. October 12, 2022. the centers for disease control and prevention (cdc) encourages those eligible to receive the new bivalent covid 19 booster vaccine. "the bivalent booster vaccine is a new vaccine that provides protection against the original strain of covid 19, as well as the two more recent variants ba.4 and ba.5," says dr. nipunie rajapakse.
Full Fda Approval For The Pfizer Covid 19 Vaccine Could Come As Soon As

Comments are closed.