The Purpose Of Clinical Trials
How To Conduct Human Clinical Trials Biopharma Services Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current. Overall purpose of clinical trials. the purpose of clinical trials is to find ways to more effectively prevent, diagnose, or treat disease. every medication, vaccine, and procedure that is used in modern medicine was once studied as a part of a clinical trial. myths about clinical trials abound—such as you will be essentially a human guinea pig.

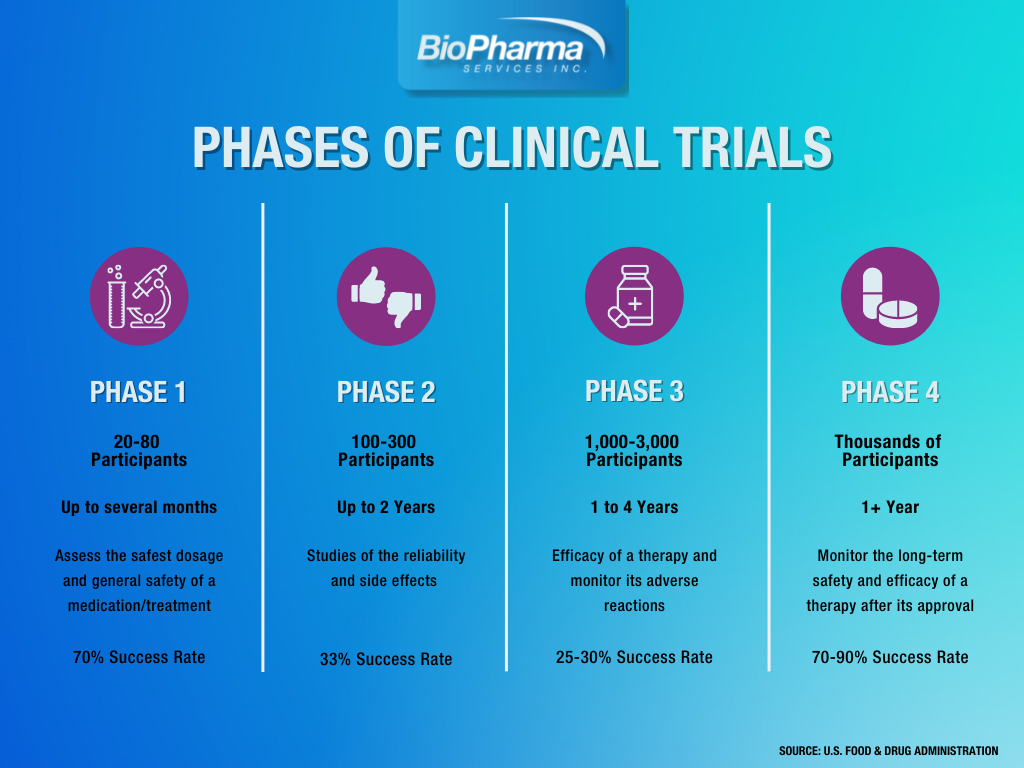
Clinical Trials Directory Ut Medical Center Clinical research is the study of health and illness in people. there are two main types of clinical research: observational studies and clinical trials. read and share this infographic (pdf, 317k) to learn why researchers do different kinds of clinical studies. observational studies monitor people in normal settings. Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes. people volunteer to take part in clinical trials to test medical interventions including drugs, cells and other biological products, surgical procedures, radiological procedures, devices, behavioural treatments and preventive care. Clinical trials are conducted in phases. each phase has a different purpose and helps researchers answer different questions. phase i trials: researchers test an experimental drug or treatment in a small group of people (20–80) for the first time. the purpose is to evaluate its safety and identify side effects. Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. clinical research is different than laboratory research. it involves people who volunteer to help us better understand medicine and health. lab research generally does not involve people — although it helps us learn.
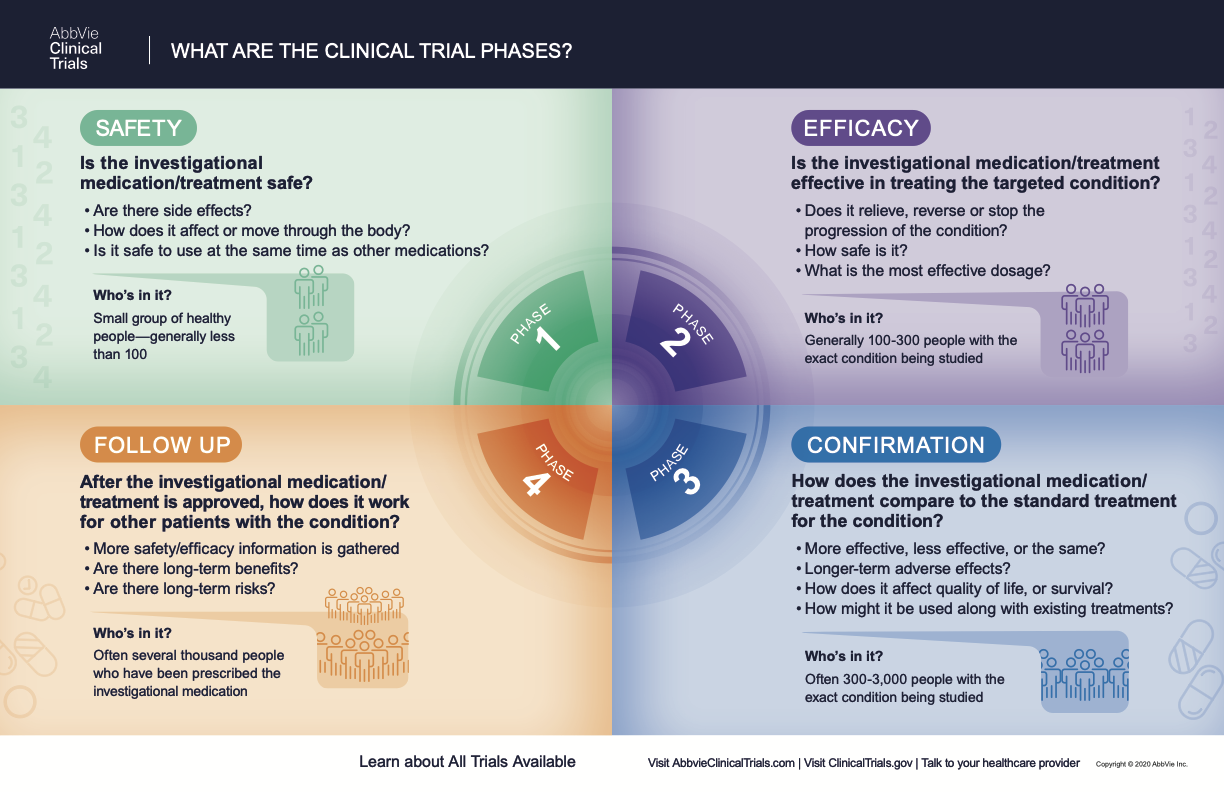
What Are The Phases Of Clinical Trials Clinical Trial Phases Clinical trials are conducted in phases. each phase has a different purpose and helps researchers answer different questions. phase i trials: researchers test an experimental drug or treatment in a small group of people (20–80) for the first time. the purpose is to evaluate its safety and identify side effects. Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. clinical research is different than laboratory research. it involves people who volunteer to help us better understand medicine and health. lab research generally does not involve people — although it helps us learn. Clinical trials are an important part of medical research aimed at learning how best to prevent, diagnose, and treat various health conditions. the purpose of this phase is to gather more. People who take part in clinical research make it possible for this to occur. the path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. the purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science. informed consent.

Comments are closed.