Titration Of Kmno4 Vs Ferrous Ammonium Sulphate Redox Titration How To

Titration Of Kmno4 Vs Ferrous Ammonium Sulphate Redox Titration How To Procedure: (a) preparation of 0.05m standard solution of ferrous ammonium sulfate: the quantity of mohr’s salt required for the 250ml of the solution having a normality of 0.05n can be calculated as follows. the molar mass of mohr’s salt = 392 g mol. strength = normality x equivalent weight. = (1 20) x 392 = 19.6 g l. In this video redox titration of ferrous ammonium sulphate (fas) vs kmno4 is performed in this video and related points like: how to prepare fas solution? wh.
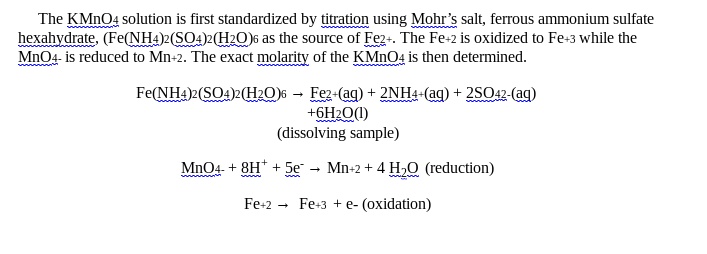
Solved The Kmno4 Solution Is First Standardized By Titration Using In the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is colourless to light pink. most laboratory manuals and books advice us to check whether the light pink colour stays or not for 30 seconds. if the colour stays after 30 seconds, then end point is reached. If a redox titration is to be used in a quantitative analysis, the titrand must initially be present in a single oxidation state. for example, iron can be determined by a redox titration in which ce 4 oxidizes fe 2 to fe 3 . depending on the sample and the method of sample preparation, iron may initially be present in both the 2 and 3. 10ml of ferrous ammonium sulfate (mohr’s salt) sulfuric acid is poured into the conical flask. kmno4 acts as a self indicator. colourless to permanent pale pink colour is the endpoint. (image will be uploaded soon) a. preparation of 250ml of m 20 solution of mohr’s salt –. the molar mass of mohr’s salt is 392gmol 1. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate, (fe(nh4)2(so4)2(h2o)6 as the source of fe2 . the fe 2 is oxidized to fe 3 while the mno4 is reduced to mn 2. the exact molarity of the kmno4 is then determined. next the standardized kmno4 is reacted with a known mass.

Comments are closed.