Titration Of Kmno4 Vs Mohr Salt Find Out Concentration Of Kmno4
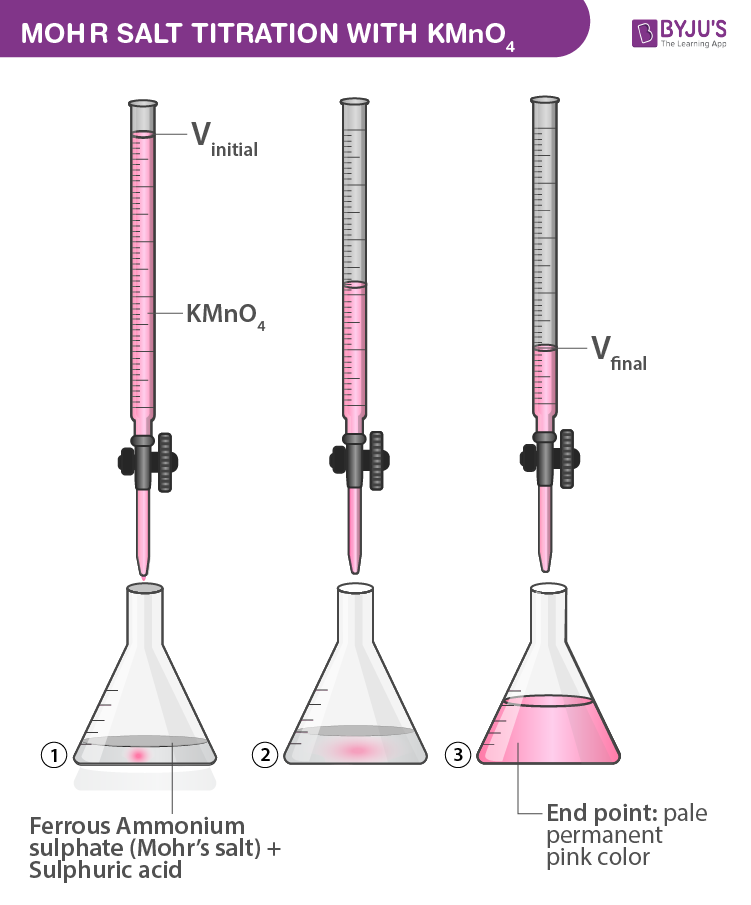
Mohr Salt Titration With Kmno4 Cbse Chemistry Practicals Class 12 Procedure: (a) preparation of 0.05m standard solution of ferrous ammonium sulfate: the quantity of mohr’s salt required for the 250ml of the solution having a normality of 0.05n can be calculated as follows. the molar mass of mohr’s salt = 392 g mol. strength = normality x equivalent weight. = (1 20) x 392 = 19.6 g l. Fill the burette with potassium permanganate solution. take a conical flask and add 5ml of dilute sulfuric acid to it. pipette out 10 ml of prepared standard mohr’s salt solution in the same conical flask. place a white tile under the burette and place the conical flask containing mohr’s salt solution and h2so4 on it.

Titration Kmno4 Vs Mohr S Salt Full Experiment Calculation Youtube Mohr salt, on the other hand, is a double salt that forms a single crystalline structure with the formula feso 4.(nh 4) 2 so 4.6h 2 o, also known as ferrous ammonium sulfate. mohr salt serves as a reducing agent in this titration, while potassium permanganate acts as an oxidising agent. Here i am explaining , how to do titration of mohr’s salt (ferrous ammonium sulphate) with kmno4 solution. the solution whose concentration is known is called standard solution. the concentration of solution which has to be determined is called unknown solution .unknown solution is added slowly to a certain volume of the standard solution. What is the function of sulphuric acid in the titration of mohr salt against kmno4? answer. the most basic role of sulphuric acid in the redox titration of the mohr salt against potassium permanganate is to prevent the hydrolysis of the ferric ion (fe 2 ) because the titration occurs in the presence of kmno 4 or k 2 cr 2 o 7 , both of which. Join me on telegram through t.me aparnabhardwajwatch all other videos on titration class xii follow the given links.1.for learning calculation on titr.

Titration Of Kmno4 With Mohr S Salt Class 12 Determine The What is the function of sulphuric acid in the titration of mohr salt against kmno4? answer. the most basic role of sulphuric acid in the redox titration of the mohr salt against potassium permanganate is to prevent the hydrolysis of the ferric ion (fe 2 ) because the titration occurs in the presence of kmno 4 or k 2 cr 2 o 7 , both of which. Join me on telegram through t.me aparnabhardwajwatch all other videos on titration class xii follow the given links.1.for learning calculation on titr. Mohr salt titration with kmno4. titration is a method used in analytical chemistry to determine the concentration of a solution by reacting it with a known volume and concentration of another solution. in the titration of mohr salt with kmno 4, a standard solution of kmno 4 is slowly added to a solution of mohr's salt until the reaction is. The titration of potassium permanganate (kmno4) against mohr salt is an example of a redox titration. in this titration, kmno4 acts as a strong oxidizing agent in the presence of sulfuric acid, oxidizing the ferrous ions in mohr salt. the reaction proceeds until the pink color of kmno4 is no longer discharged. this determines the endpoint of the titration, allowing the molarity and strength of.

Comments are closed.