Titration Of Kmno4 With Mohr S Salt Class 12 Determine The
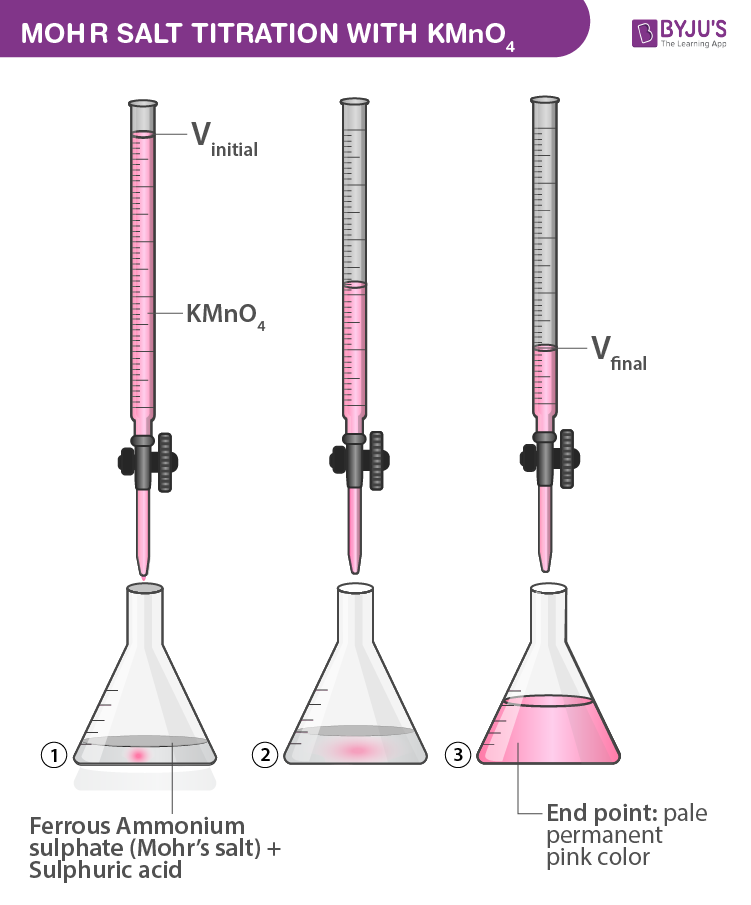
Mohr Salt Titration With Kmno4 Cbse Chemistry Practicals Class 12ођ Procedure: (a) preparation of 0.05m standard solution of ferrous ammonium sulfate: the quantity of mohr’s salt required for the 250ml of the solution having a normality of 0.05n can be calculated as follows. the molar mass of mohr’s salt = 392 g mol. strength = normality x equivalent weight. = (1 20) x 392 = 19.6 g l. In this animated video, you will learn titration of kmno4 with mohr's salt class 12. by this experiment, you determine the concentration of kmno4 using mohr.

Titration Of Kmno4 With Mohr S Salt Class 12 Determine The 10ml of ferrous ammonium sulfate (mohr’s salt) sulfuric acid is poured into the conical flask. kmno4 acts as a self indicator. colourless to permanent pale pink colour is the endpoint. (image will be uploaded soon) a. preparation of 250ml of m 20 solution of mohr’s salt –. the molar mass of mohr’s salt is 392gmol 1. Join me on telegram through t.me aparnabhardwajwatch all other videos on titration class xii follow the given links.1.for learning calculation on titr. 1. fill the burette with kmno4 solution. 2. pipette out 10ml. of mohr’s salt solution into the conical flask. 3. add half a test tube of diluted h2so4. 4. keep a glazed tile under the burette and place the conical flask on it. 5. Mohr salt, on the other hand, is a double salt that forms a single crystalline structure with the formula feso 4.(nh 4) 2 so 4.6h 2 o, also known as ferrous ammonium sulfate. mohr salt serves as a reducing agent in this titration, while potassium permanganate acts as an oxidising agent.

Comments are closed.