Transition Elements General Properties And Trends With Faqs 2022
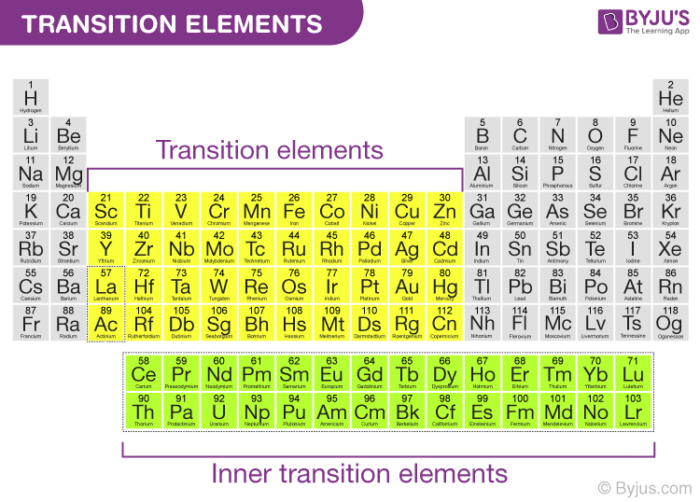
Transition Elements General Properties And Trends With Faqs 2022 Iupac defines transition elements as an element having a d subshell that is partially filled with electrons, or an element that has the ability to form stable cations with an incompletely filled d orbital. in general, any element which corresponds to the d block of the modern periodic table (which consists of groups 3 12) is considered to be a. Most compounds of transition metals are paramagnetic, whereas virtually all compounds of the p block elements are diamagnetic. the electronegativities of the first row transition metals increase smoothly from sc (χ = 1.4) to cu (χ = 1.9). thus sc is a rather active metal, whereas cu is much less reactive.

Transition Elements General Properties And Trends With Faqs 2022 Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. as shown in figure 23.1.2 23.1. 2, the d block elements in groups 3–11 are transition elements. the f block elements, also called inner transition metals (the lanthanides and actinides), also meet this criterion because the d orbital is. Sometimes they are referred to as transition metals; not all d block elements are classed as transition elements: scandium and zinc, for example, are not classed as transition elements, despite being in the d block; scandium is not classed as a transition element because: it only forms one ion, sc 3. Figure 22.1.1 22.1. 1 the metallic radii of the first , second , and third row transition metals. because of the lanthanide contraction, the second and third row transition metals are very similar in size. as you learned in chapter 7, electrons in ( n − 1) d and ( n − 2) f subshells are only moderately effective at shielding the nuclear. Mxenes are a family of two dimensional materials of great interest due to their unique properties, e.g., adjustability based on changes in their composition, structure, and surface functionality, which grant mxenes a variety of applications. one way of changing the catalytic effect of mxenes consists of adsorbing isolated metallic elements, such as transition metals (tms), onto their surface.
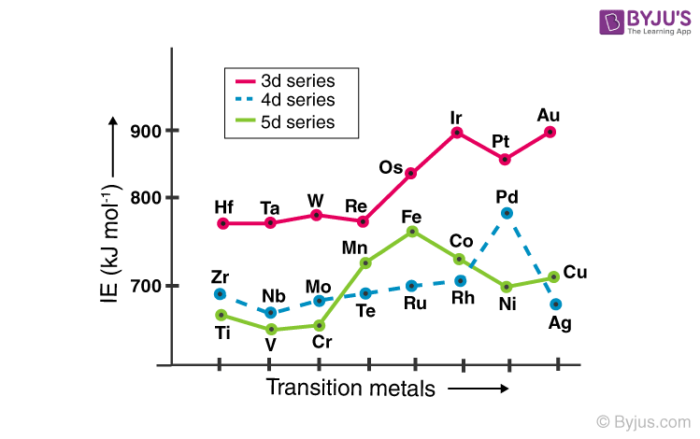
Transition Elements General Properties And Trends With Faqs 2022 Figure 22.1.1 22.1. 1 the metallic radii of the first , second , and third row transition metals. because of the lanthanide contraction, the second and third row transition metals are very similar in size. as you learned in chapter 7, electrons in ( n − 1) d and ( n − 2) f subshells are only moderately effective at shielding the nuclear. Mxenes are a family of two dimensional materials of great interest due to their unique properties, e.g., adjustability based on changes in their composition, structure, and surface functionality, which grant mxenes a variety of applications. one way of changing the catalytic effect of mxenes consists of adsorbing isolated metallic elements, such as transition metals (tms), onto their surface. If we go through the orbital theory then these metals can be defined as the elements that have half filled d orbitals, in the periodic table d block elements from the groups 3–12 are transition elements, and the f block elements, are called inner transition elements definition i.e. the lanthanides and actinides, also possess partially occupied d orbital before the f orbitals. Transition metal definition. the most common definition of a transition metal is the one accepted by the iupac. a transition metal is an element with a partially filled d subshell or the capacity to produce cations with an incomplete d subshell. other people consider the transition metals to include any d block element on the periodic table.

Comments are closed.