Transition Metals Chemistry Learner

Transition Metals Chemistry Learner Transition metals are a group of metals in the middle of the periodic table that have some similar properties to one another. a unique feature of these metals is that they have valence electrons in two shells, and electrons from both these shells participate in chemical bonding. as a result, they show several common oxidation states [1]. A fundamental property of d block metals (aka transition metals) is that they are predisposed to form coordination complexes, which have a metal in the middle that is surrounded by ions or atoms (aka ligands). these coordination complexes have special properties, which are described in detail in lectures 28 and 29.
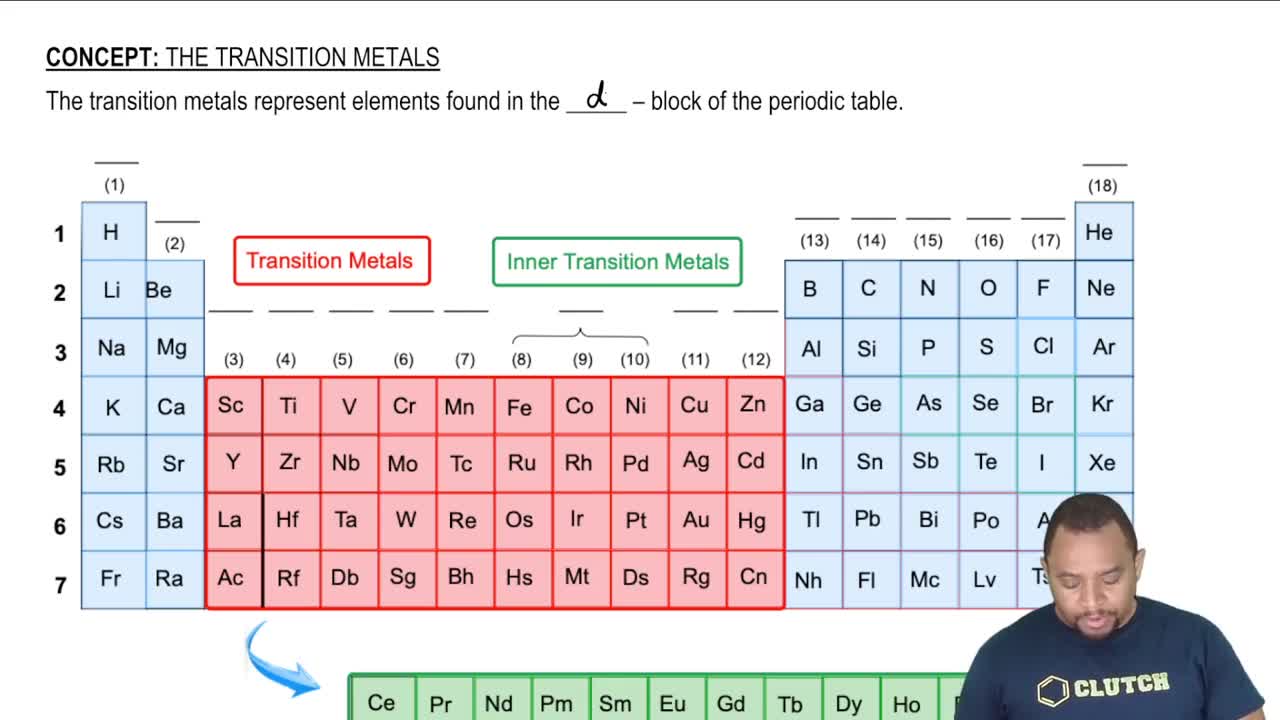
Transition Metals Periodic Table Chemistry Khan Academy Actinium, ac, is the first member of the fourth transition series, which also includes rf through rg. figure 19.1.2 19.1. 2: the transition metals are located in groups 3–11 of the periodic table. the inner transition metals are in the two rows below the body of the table. A transition metal is one that forms one or more stable ions which have incompletely filled d orbitals. on the basis of this definition, scandium and zinc do not count as transition metals even though they are members of the d block. scandium has the electronic structure [ar] 3d 1 4s 2. Introduction to transition metals i. the elements of the second and third rows of the periodic table show gradual changes in properties across the table from left to right as expected. electrons in the outer shells of the atoms of these elements have little shielding effects resulting in an increase in effective nuclear charge due to the. Description: embedded video, no tabs, this description appears on section page: a fundamental property of d block metals (aka transition metals) is that they are predisposed to form coordination complexes, which have a metal in the middle that is surrounded by ions or atoms (aka ligands). these coordination complexes have special properties.

Comments are closed.