Transition Metals Periodic Table Chemistry Khan Academy
Transition Metals Periodic Table Chemistry Khan Academy The definition of a transition metal, and how to write the electron configuration including examples for fe and zn. created by jay.watch the next lesson: htt. Khanmigo is now free for all us educators! plan lessons, develop exit tickets, and so much more with our ai teaching assistant.

Transition Metals Periodic Table Chemistry Khan Academy Youtube Discover the patterns and trends of the elements in the periodic table with this engaging video from khan academy, a free online learning platform. Transition metals and coordination compounds 3h 16m. worksheet. atomic radius & density of transition metals. electron configurations of transition metals. electron configurations of transition metals: exceptions. paramagnetism and diamagnetism. ligands. complex ions. coordination complexes. Courses on khan academy are always 100% free. start practicing—and saving your progress—now! khanacademy.org science hs chemistry x2613d8165d88df. With the main group elements covered, it's time to check out the other sections of the periodic table, those being the transition metals, as well as the lant.

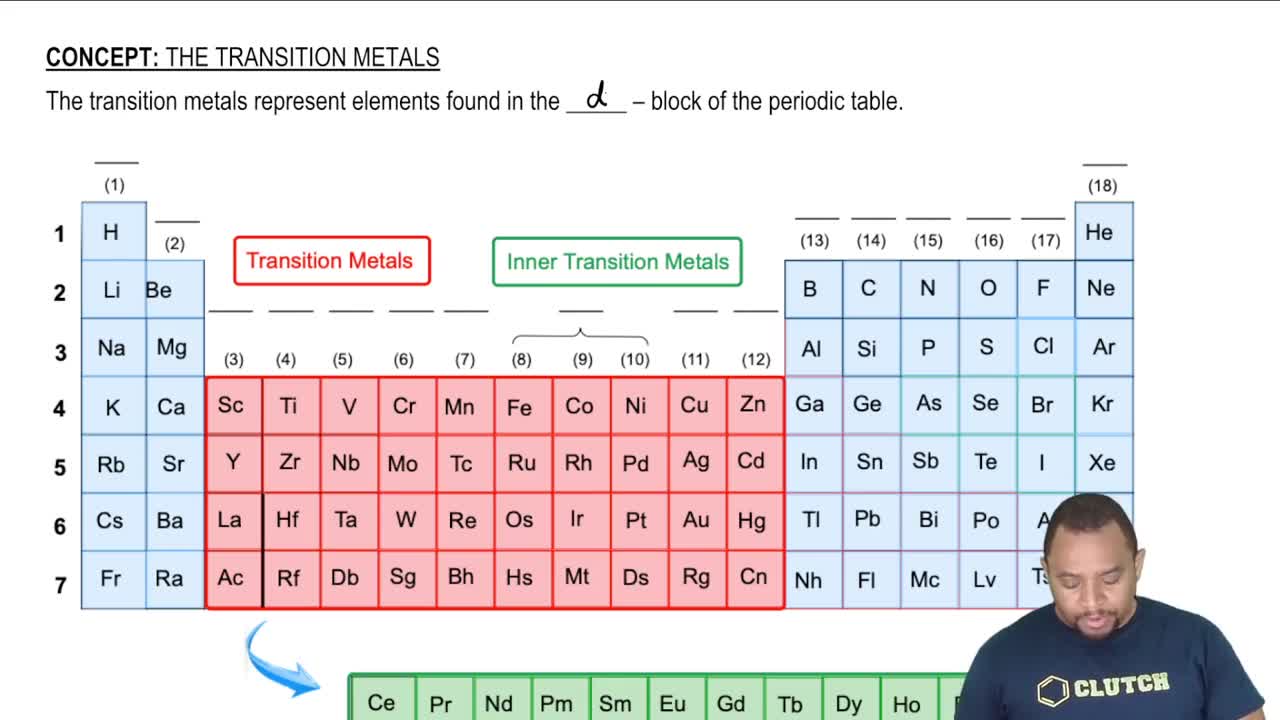
The Periodic Table Transition Metals Periodic Table Chemistry Courses on khan academy are always 100% free. start practicing—and saving your progress—now! khanacademy.org science hs chemistry x2613d8165d88df. With the main group elements covered, it's time to check out the other sections of the periodic table, those being the transition metals, as well as the lant. The transition metals are the largest group of elements on the periodic table. they got their name because english chemist charles bury described a transition series of elements in 1921. bury examined the transition from an inner electron layer with 8 electrons to a layer with 18 electrons and from a layer of 18 electrons to one with 32. The elements can also be classified into the main group elements (or representative elements) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; 1 and inner transition metals in the two rows at the bottom of the table (the top row elements are called lanthanides and the bottom row elements are.

Transition Metals Chemistry Learner The transition metals are the largest group of elements on the periodic table. they got their name because english chemist charles bury described a transition series of elements in 1921. bury examined the transition from an inner electron layer with 8 electrons to a layer with 18 electrons and from a layer of 18 electrons to one with 32. The elements can also be classified into the main group elements (or representative elements) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; 1 and inner transition metals in the two rows at the bottom of the table (the top row elements are called lanthanides and the bottom row elements are.

Comments are closed.