Types Of Bonding Ionic Covalent Metallic Gcse Chemistry Revision
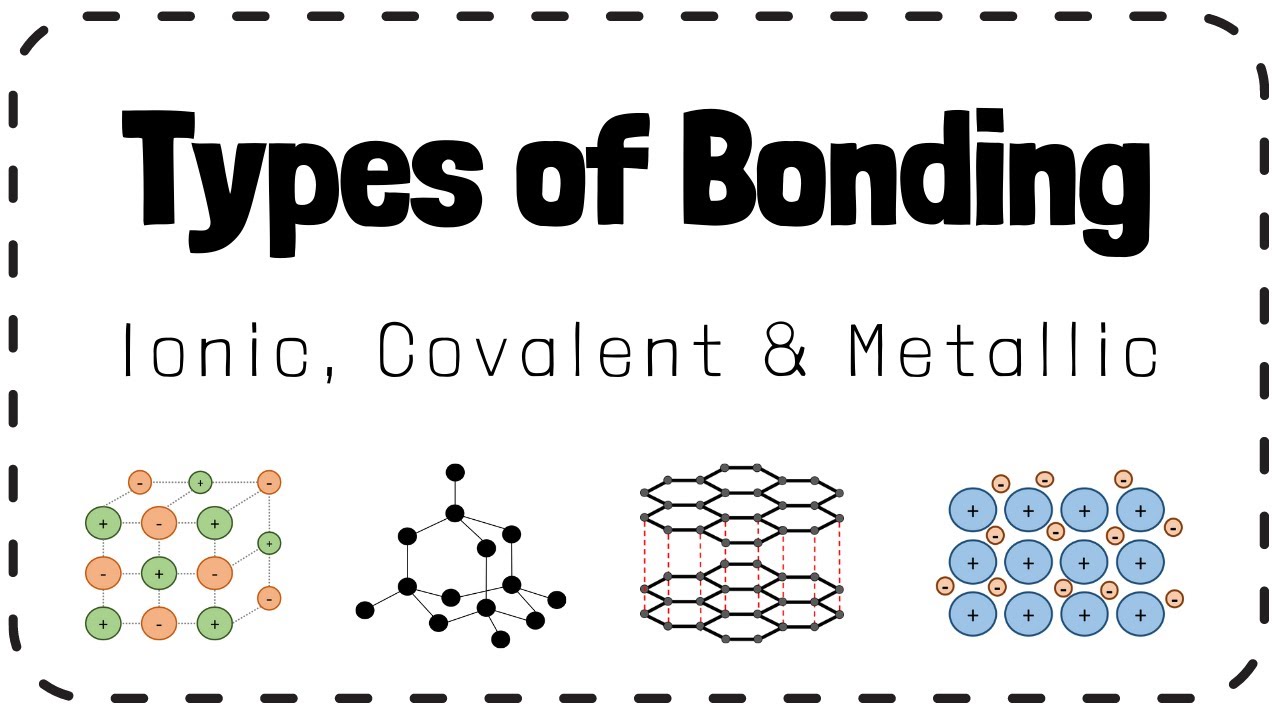
Types Of Bonding Ionic Covalent Metallic Gcse Chemistry Revision Which gcse chemistry science t hi everyone,i hope this video helps you to feel more confident with identifying and describing the different types of bonding. There are three types of bonding studied at gcse. ionic bonding. covalent bonding. metallic bonding. ionic bonds: takes place when metals and non metals react by transferring electrons. the atoms involved are oppositely charged particles (known as ions) in which electron transfer occurs. the opposite charges attract through electrostatic forces.

Gcse Chemistry Topic 2a The Bonding Structure Of Elements Youtube Gcse; wjec; bonding ionic bonding. when a metal element reacts with a non metal element an ionic compound is formed. when a non metal element reacts with a non metal element a covalent bond is formed. There are three types of strong chemical bonds: ionic, covalent and metallic. for ionic bonding the particles are oppositely charged ions . for covalent bonding the particles are atoms which share pairs of electrons. for metallic bonding the particles are atoms which share delocalised electrons. ionic bonding occurs in compounds formed from. Individual metal atoms are held together by strong metallic bonds forming a lattice structure. this type of bonding occurs in metals and metal alloys, which are mixtures of metal. within the metal lattice, the atoms lose their valence electrons and become positively charged metal ions. the valence electrons no longer belong to any specific. There are three types of bonding studied at gcse: ionic bonding an ionic bond is the attraction between oppositely charged ions. covalent bonding a covalent bond is formed by a shared pair of.

Gcse 1 9 Chemistry Ionic Metallic And Covalent Bonding Typesо Individual metal atoms are held together by strong metallic bonds forming a lattice structure. this type of bonding occurs in metals and metal alloys, which are mixtures of metal. within the metal lattice, the atoms lose their valence electrons and become positively charged metal ions. the valence electrons no longer belong to any specific. There are three types of bonding studied at gcse: ionic bonding an ionic bond is the attraction between oppositely charged ions. covalent bonding a covalent bond is formed by a shared pair of. Metallic bonding involves an attraction between positively charged ions and negatively charged delocalised electrons. metallic bonds are found in metals and alloys (mixtures of metals and other substances). atoms can chemically bond (join together) in 3 ways: ionic bonding, covalent bonding or metallic bonding. Compounds are substances in which atoms of two, or more, elements are not just mixed together but chemically combined. this is known as bonding. chemical reactions between elements involve either the giving and taking, or sharing, of electrons in the highest occupied energy levels of atoms. this video explains about bonding and the different.

Ionic Covalent Metallic Bonding I Gcse Chemistry Detailed Notes Metallic bonding involves an attraction between positively charged ions and negatively charged delocalised electrons. metallic bonds are found in metals and alloys (mixtures of metals and other substances). atoms can chemically bond (join together) in 3 ways: ionic bonding, covalent bonding or metallic bonding. Compounds are substances in which atoms of two, or more, elements are not just mixed together but chemically combined. this is known as bonding. chemical reactions between elements involve either the giving and taking, or sharing, of electrons in the highest occupied energy levels of atoms. this video explains about bonding and the different.

Comments are closed.