Types Of Matter Elements Compounds And Mixtures

1 8 Elements Compounds Mixtures вђ Chemistry Matter can be broken down into two categories: pure substances and mixtures. pure substances are further broken down into elements and compounds. mixtures are physically combined structures that can be separated into their original components. a chemical substance is composed of one type of atom or molecule. a mixture is composed of different. What's the difference between a physical change and a chemical change? what are elements, compounds, pure substances, and mixtures? so many definitions to le.
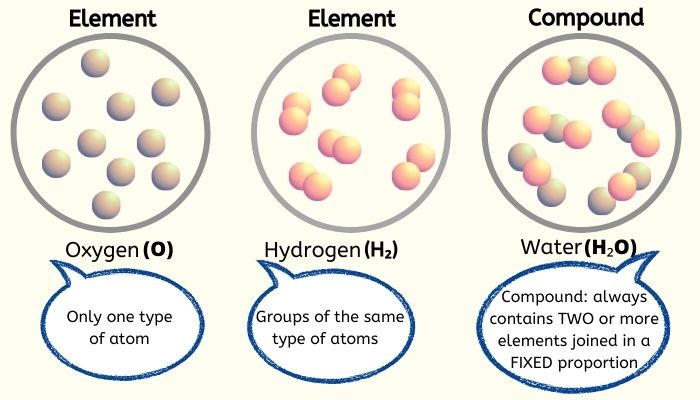
States Of Matter Elements Compounds And Mixtures Atomic Structure My This chemistry video tutorial provides a basic introduction into the different types of matter such as elements, compounds, mixtures, and pure substances.che. Figure 3.4.1 3.4. 1: relationships between the types of matter and the methods used to separate mixtures. ordinary table salt is called sodium chloride. it is considered a substance because it has a uniform and definite composition. all samples of sodium chloride are chemically identical. water is also a pure substance. Figure 2.2.2.1 2.2.2. 1: the classification of matter. matter can be classified in a variety of ways, depending on its properties. elements and compounds are both examples of pure substances. a substance that cannot be broken down into chemically simpler components is an element. oxygen, o, and hydrogen, h, are each examples of elements. Modified by joshua halpern ( howard university) is shared under a license and was authored, remixed, and or curated by libretexts. matter can be classified according to physical and chemical properties. matter is anything that occupies space and has mass. the three states of matter are solid, liquid, and gas. a physical change ….

Types Of Matter Elements Compounds And Mixtures Matter And Its Figure 2.2.2.1 2.2.2. 1: the classification of matter. matter can be classified in a variety of ways, depending on its properties. elements and compounds are both examples of pure substances. a substance that cannot be broken down into chemically simpler components is an element. oxygen, o, and hydrogen, h, are each examples of elements. Modified by joshua halpern ( howard university) is shared under a license and was authored, remixed, and or curated by libretexts. matter can be classified according to physical and chemical properties. matter is anything that occupies space and has mass. the three states of matter are solid, liquid, and gas. a physical change …. Close. compound a pure substance made from two or more elements which are chemically bonded in a fixed ratio. and. mixtures. close. mixture when two or more compounds or elements are present. Mixtures. microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). note that a mixture: consists of two or more different elements and or compounds physically intermingled, can be separated into its components by physical means, and. often retains many of the properties of its components.
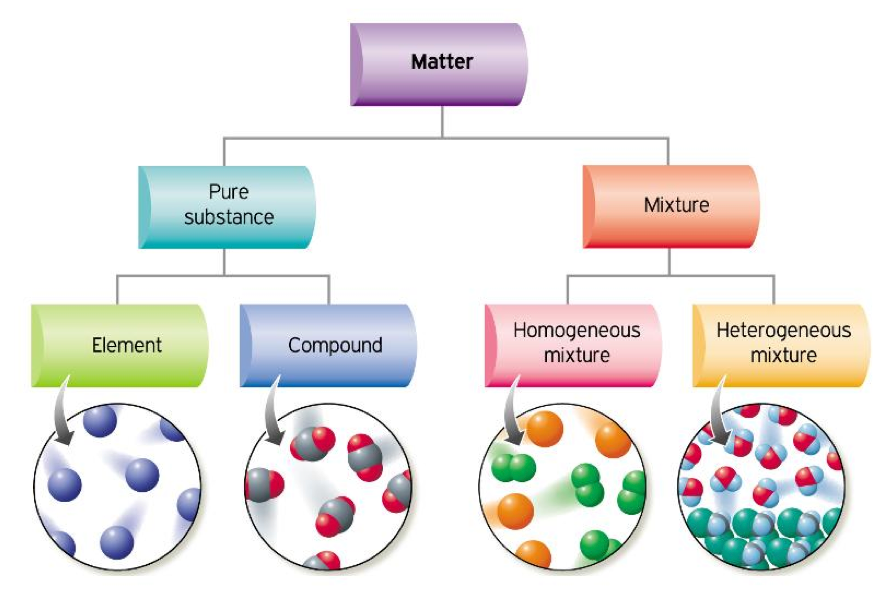
Properties Of Mixtures Aca Grade 8 Science Close. compound a pure substance made from two or more elements which are chemically bonded in a fixed ratio. and. mixtures. close. mixture when two or more compounds or elements are present. Mixtures. microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water). note that a mixture: consists of two or more different elements and or compounds physically intermingled, can be separated into its components by physical means, and. often retains many of the properties of its components.
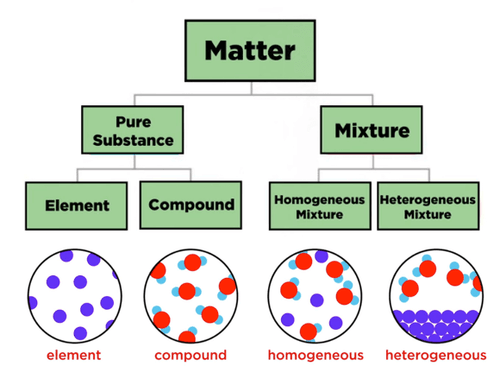
Difference Between Mixture And Compound Protonstalk

Comments are closed.