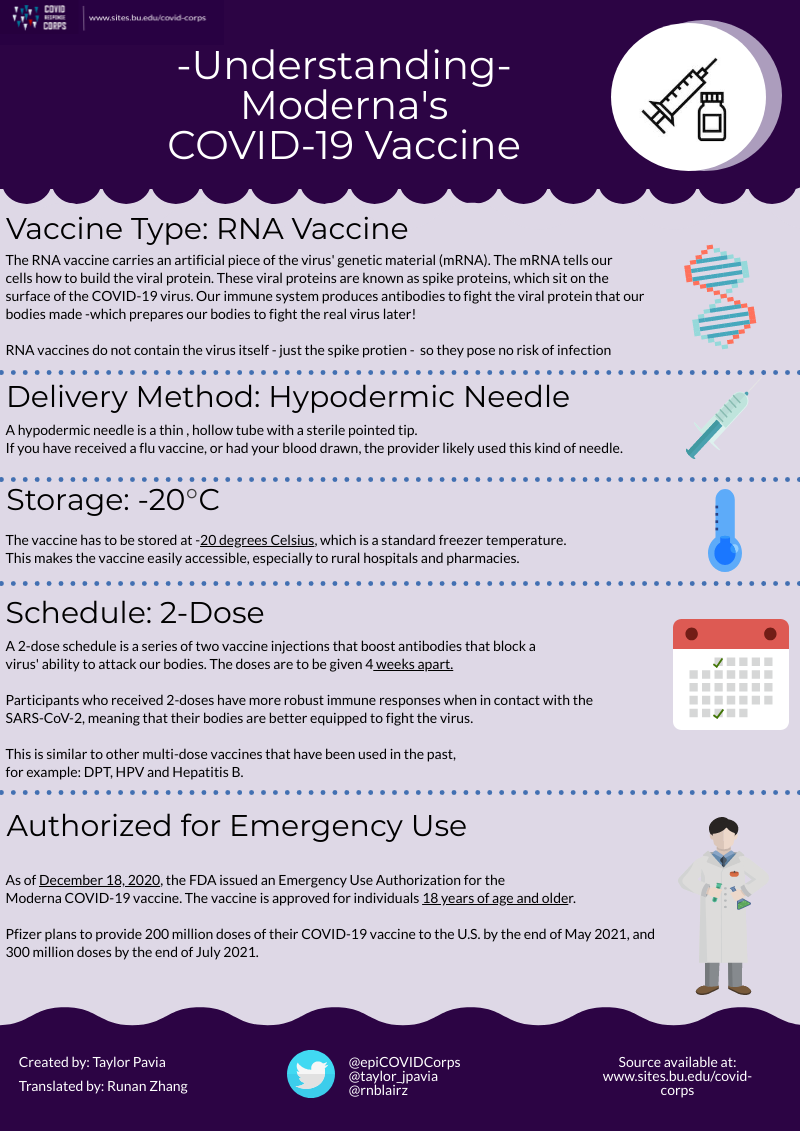
Understanding The New Vaccines Epidemiology Covid 19 Response Corps

Understanding The New Vaccines Epidemiology Covid 19 Response Corps Three covid 19 vaccines have been approved for emergency use as of march 2021: mrna vaccines produced by moderna and pfizer biontech, and an adenovirus vaccine produced by johnson and johnson. on november 9th, 2020, pfizer announced that their vaccine candidate was found to be more than 90% effective in preventing covid 19 infections. Where 2020 saw the development and testing of vaccines against severe acute respiratory syndrome coronavirus 2 (sars cov 2) at an unprecedented pace, the first half of 2021 has seen vaccine.
Understanding The New Vaccines Epidemiology Covid 19 Response Corps Nature medicine 27 , 1338–1339 ( 2021) cite this article. a new study unpacks the complexities of covid 19 vaccine hesitancy and acceptance across low , middle and high income countries. as of. The uncertainty and constantly evolving nature of the covid 19 pandemic and response measures, the rapid introduction of new vaccines, emerging variants, and the volatility of the surrounding. 3.4. covid 19 vaccine effectiveness and disease management. the initial covid 19 vaccines developed (bnt162b2, mrna 1273, chadox1, and ad26.cov2 s), were designed to target the s protein of the ancestral wuhan 1 strain and were highly effective at preventing symptomatic sars cov 2 infections in clinical trials [citation 58]. however. The effectiveness of the mrna vaccines in preventing covid 19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. efficacy observed in the trials was more than 90%.1,2 the efficacy of other vaccines evaluated in large randomised trials, such as the oxford–astrazeneca (70%) and sputnik v (91%) vaccines, have been criticised for elements.
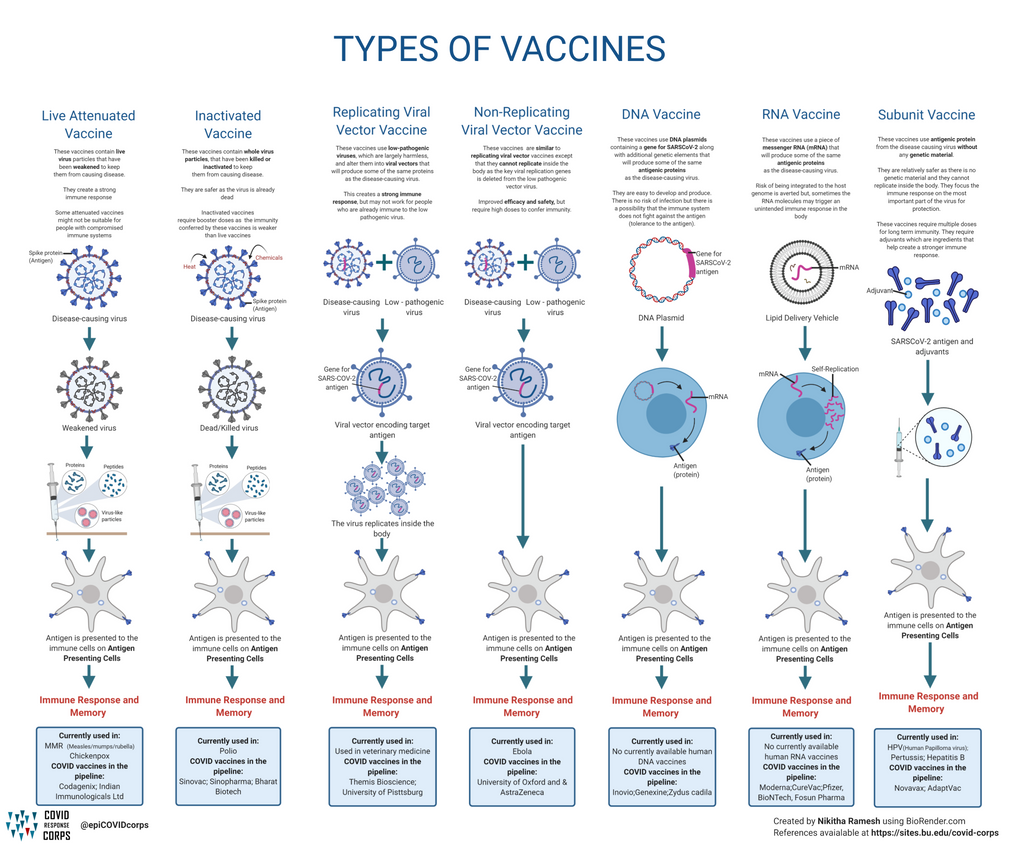
Types Of Vaccines Infographics Epidemiology Covid 19 Response Corps 3.4. covid 19 vaccine effectiveness and disease management. the initial covid 19 vaccines developed (bnt162b2, mrna 1273, chadox1, and ad26.cov2 s), were designed to target the s protein of the ancestral wuhan 1 strain and were highly effective at preventing symptomatic sars cov 2 infections in clinical trials [citation 58]. however. The effectiveness of the mrna vaccines in preventing covid 19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. efficacy observed in the trials was more than 90%.1,2 the efficacy of other vaccines evaluated in large randomised trials, such as the oxford–astrazeneca (70%) and sputnik v (91%) vaccines, have been criticised for elements. Understanding the protection conferred by previous infection against repeat infection, illness, and severe disease is key to projecting the future epidemiology of covid 19 and to guiding vaccine policy decisions. in the lancet, the covid 19 forecasting team1 report data from a systematic review and meta analysis of 65 studies from 19 different countries estimating the reduction in covid 19. On 11 december 2020, the fda authorized the pfizer biontech vaccine for emergency use for individuals aged 16 years and older in the usa. this is the first covid 19 vaccine approved by the fda . the european medicines agency (ema) has also approved the pfizer biontech vaccine as the first covid 19 vaccine to be used in eu countries .

Comments are closed.