Unit Cell Chemistry Simple Cubic Body Centered Cubic Face Centered Cubic Crystal Lattice Structu

Unit Cell Chemistry Simple Cubic Body Centered Cubic This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures. it highlights the key differences between the sim. In the previous section, we identified that unit cells were the simplest repeating unit of a crystalline solid and examined the most basic unit cell, the primitive cubic unit cell. in this section, we continue by looking at two other unit cell types, the body centered cubic and the face centered cubic unit cells.
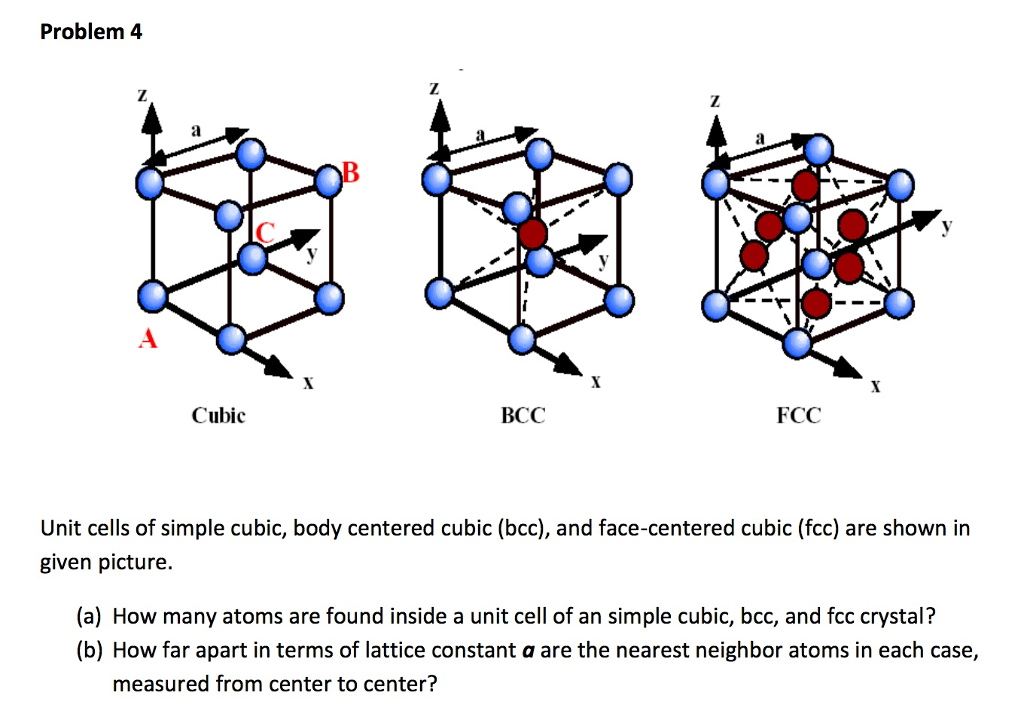
Solved Unit Cells Of Simple Cubic Body Centered Cubic B Ch And since each simple cubic unit cell has one atom at each of its eight “corners,” there is 8 × 1 8 = 1 8 × 1 8 = 1 atom within one simple cubic unit cell. figure 4.1.4 4.1. 4: a simple cubic lattice unit cell contains one eighth of an atom at each of its eight corners, so it contains one atom total. most metal crystals are one of the. Face centered cubic. figure\(\pageindex{5}\): unit cell for face centered unit cell, and diagram of cubic close packed structure that it results in. note each row has neighbors shifted from the cartesian coordinate of their plan, and as you move up the lattice there is an abcabcabc stacking , where every third layer is aligns. Body centered cubic unit cells. body centered cubic (bcc) unit cells indicate where the lattice points appear not only at the corners but in the center of the unit cell as well. the atoms touch one another along the cube's diagonal crossing, but the atoms don't touch the edge of the cube. all atoms are identical. Most metal crystals are one of the four major types of unit cells. for now, we will focus on the three cubic unit cells: simple cubic (which we have already seen), body centered cubic unit cell, and face centered cubic unit cell —all of which are illustrated in figure 10.50. (note that there are actually seven different lattice systems, some.

Unit Cell вђ Overview Examples Expii Body centered cubic unit cells. body centered cubic (bcc) unit cells indicate where the lattice points appear not only at the corners but in the center of the unit cell as well. the atoms touch one another along the cube's diagonal crossing, but the atoms don't touch the edge of the cube. all atoms are identical. Most metal crystals are one of the four major types of unit cells. for now, we will focus on the three cubic unit cells: simple cubic (which we have already seen), body centered cubic unit cell, and face centered cubic unit cell —all of which are illustrated in figure 10.50. (note that there are actually seven different lattice systems, some. Unit cells: nacl and zns. nacl should crystallize in a cubic closest packed array of cl ions with na ions in the octahedral holes between planes of cl ions. we can translate this information into a unit cell model for nacl by remembering that the face centered cubic unit cell is the simplest repeating unit in a cubic closest packed structure. Face centered cubic cells. many other metals, such as aluminum, copper, and lead, crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and at the centers of each face, as illustrated in figure 3. this arrangement is called a face centered cubic (fcc) solid. a fcc unit cell contains four atoms: one eighth of.
, bcc(body centered cubic) and fcc(face centered cubic).jpg)
Cubic Crystal Lattices Of Sc Simple Cubic Bcc Body Centeredођ Unit cells: nacl and zns. nacl should crystallize in a cubic closest packed array of cl ions with na ions in the octahedral holes between planes of cl ions. we can translate this information into a unit cell model for nacl by remembering that the face centered cubic unit cell is the simplest repeating unit in a cubic closest packed structure. Face centered cubic cells. many other metals, such as aluminum, copper, and lead, crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and at the centers of each face, as illustrated in figure 3. this arrangement is called a face centered cubic (fcc) solid. a fcc unit cell contains four atoms: one eighth of.

Unit Cell Simple Cubic Body Centered Cubic Face C

Comments are closed.