Vaccines 101 All About Phase 3 Covid 19 Vaccine Clinical Trials Johnson Johnson
Va Recruiting Volunteers For Covid 19 Vaccine Phase 3 Clinical о Phase 1 testing marks the first time the vaccine is tested in a small group of adults, usually between 20 to 80 people, to evaluate its safety and measure the immune response it generates. phase 2a studies aim to determine the most effective dose, and expand the safety experience with the vaccine. janssen’s investigational vaccine candidate. At interim analysis in a phase 3, observer blinded, placebo controlled clinical trial, the mrna 1273 vaccine showed 94.1% efficacy in preventing coronavirus disease 2019 (covid 19). after emergency.
Producing Prevention How Vaccines Are Developed Online Public Health The ad26.cov2.s vaccine was highly effective against severe–critical coronavirus disease 2019 (covid 19), hospitalization, and death in the primary phase 3 efficacy analysis. we conducted the. Single dose vaccine prevented hospitalization and death across all study participants, 28 days after vaccination vaccine shown to be effective against severe critical covid 19 disease as early as seven days after vaccination, with efficacy continuing to increase eight weeks post vaccination vaccine also shown to be consistently effective against symptomatic infection, including in south africa. You should not get the janssen covid 19 vaccine if you: had a severe allergic reaction to any ingredient of this vaccine. how is the janssen covid 19 vaccine given? the janssen covid 19 vaccine will be given to you as an injection into the muscle. the janssen covid 19 vaccine vaccination schedule is a single dose. In this ongoing, double blind, randomized, placebo controlled, phase 3 clinical trial, we investigated the safety, vaccine efficacy, and immunogenicity of two doses of azd1222 as compared with.

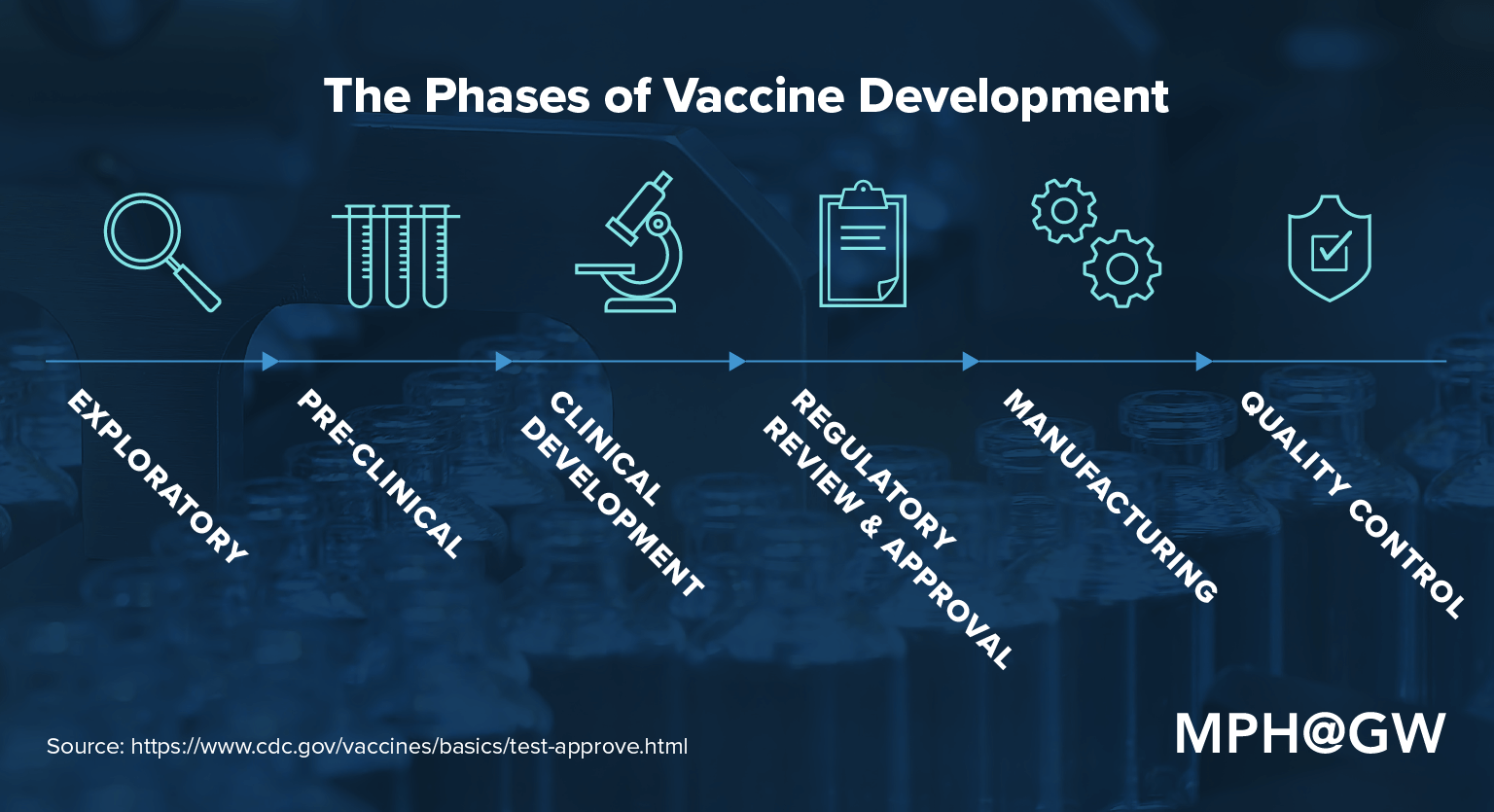
4 Phases Of Covid 19 Vaccine Clinical Trials Explained You should not get the janssen covid 19 vaccine if you: had a severe allergic reaction to any ingredient of this vaccine. how is the janssen covid 19 vaccine given? the janssen covid 19 vaccine will be given to you as an injection into the muscle. the janssen covid 19 vaccine vaccination schedule is a single dose. In this ongoing, double blind, randomized, placebo controlled, phase 3 clinical trial, we investigated the safety, vaccine efficacy, and immunogenicity of two doses of azd1222 as compared with. The phase 3 ensemble study is designed to evaluate the efficacy and safety of the janssen covid 19 vaccine candidate in protecting moderate to severe covid 19, with co primary endpoints of 14 days. The investigational vaccine directs the body’s cells to express the spike protein to elicit a broad immune response. a phase 1 clinical trial found the candidate vaccine to be safe, generally well tolerated and able to induce antibodies with high levels of virus neutralizing activity. moderna initiated phase 2 testing of the vaccine in may 2020.

Comments are closed.