Video 16 Responsibilities Of Investigator

Video 16 Responsibilities Of Investigator Youtube Quick refresher on responsibilities of investigator. Chapter 12: investigator’s role and responsibilities. revised on: 1 23 2024. this chapter defines the role of principal investigator, co investigator, and student investigator in human subjects research. additionally, it identifies the specific responsibilities, qualifications, and interactions of an investigator.
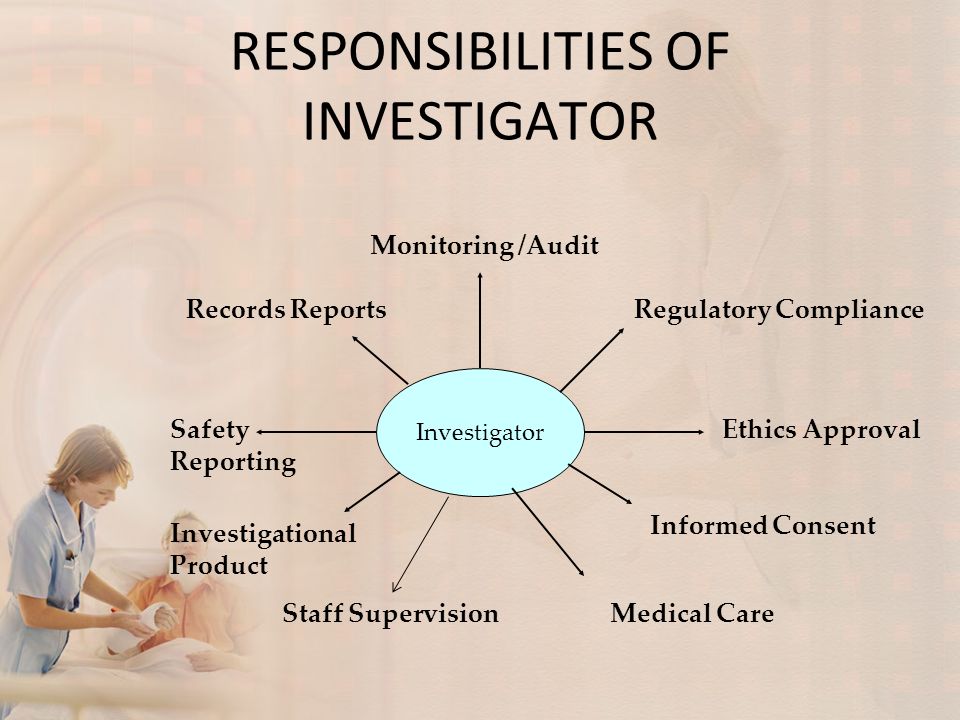
Responsibilities Of Investigator Ppt Video Online Download 4.1 investigator’s qualifications and agreements. 4.1.1 the investigator(s) should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial, should meet all the qualifications specified by the applicable regulatory requirement(s), and should provide evidence of such qualifications through up to date curriculum vitae and or other. This guidance provides an overview of the responsibilities of a person who conducts a clinical investigation of a drug, biological product, or medical device (an investigator as defined in 21 cfr. The required reports for medical devices are outlined in 21 cfr §812.150. 16 financial disclosures to the sponsor should be updated as necessary during the investigation and for 1 year following study completion. 17 having a significant equity interest in the sponsor, which is any stock in a non–publicly traded company or $50,000 of stock in. Fda guidance for industry. this section of the guidance clarifies the investigator’s responsibility to supervise the conduct of the clinical investigation and to protect the rights, safety, and welfare of participants in drug and medical device clinical trials. a. supervision of the conduct of a clinical investigation.

Comments are closed.