What Everybody Should Know About Clinical Trials Part 7 Phase 4 Trials
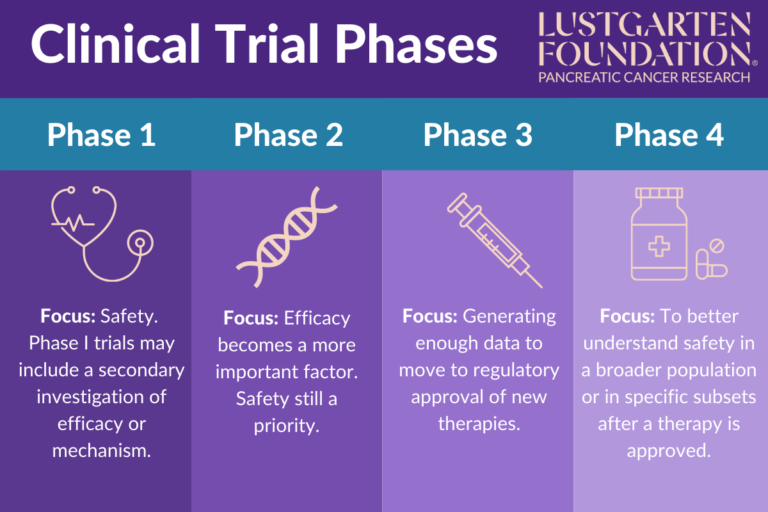
Clinical Trial Phases Without clinical trials, we wouldn’t have any vaccines, treatments for cancer, heart disease, diabetes and many others. what should everybody know about clin. The main objective of the phase 4 trial is to check the drug's performance in real life scenarios, to study the long term risks and benefits of using the drug and to discover any rare side effects.

All About Cancer Clinical Trials Trial Safety Measures Masonic There are benefits and risks to taking part in each phase of a clinical trial. although there are clinical trials for devices as well as other diseases and treatments, drugs for cancer patients are used in the examples of clinical trial phases described here. phase 0 clinical trials: exploring if and how a new drug may work. Phase 4 clinical trials. after the fda approves a treatment, it may be studied more in a phase 4 trial. we do not do phase 4 clinical trials at msk. phase 4 trials assess the side effects, risks, and benefits of a drug or other therapy. they test it over a long period of time and in a larger number of people than in phase 3 trials. These trials are rare. phase 0 trials are tiny trials that help researchers decide if a new agent should be tested in a phase 1 trial. phase 4 trials look at long term safety and effectiveness. they occur after a new treatment has been approved and is on the market. the following shows the number of patients participating and the purpose of the. The four phases of clinical trials. early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. later phases (phase 3 and 4) compare the treatment to current standard treatments. in a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects.
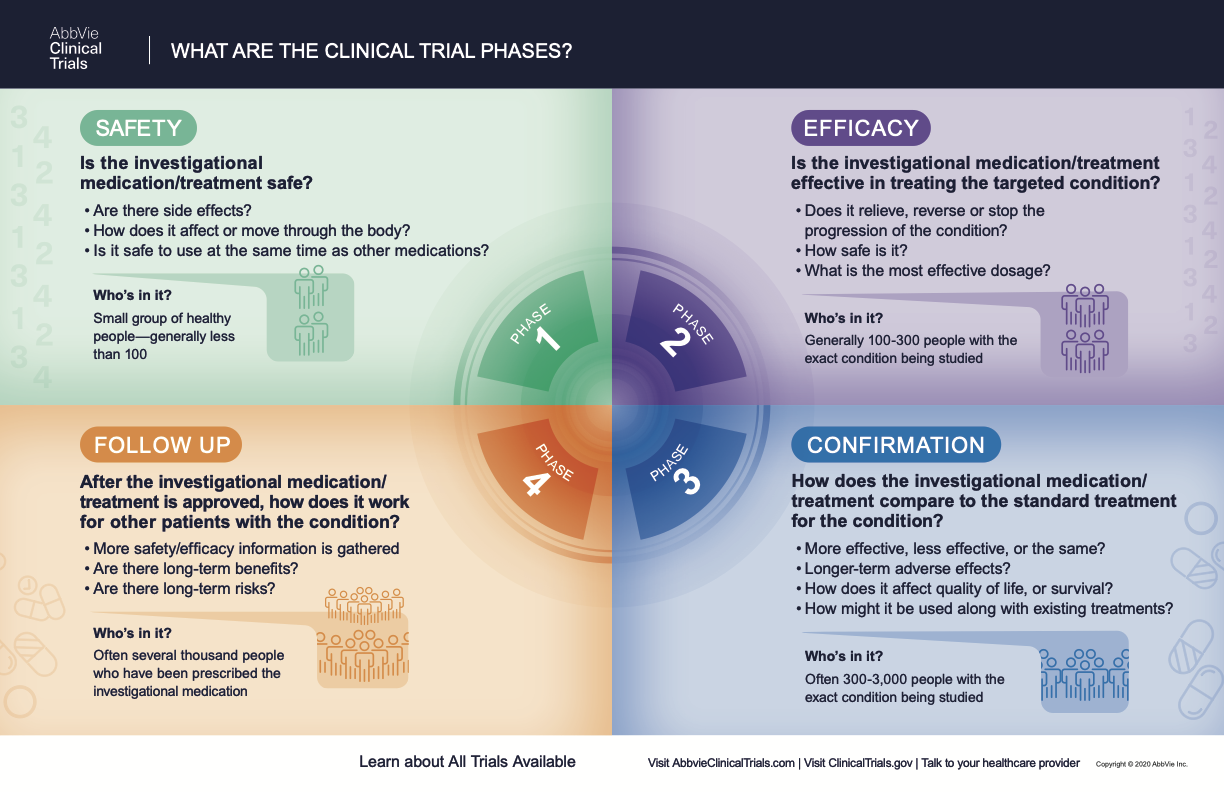
What Are The Phases Of Clinical Trials Clinical Trial Phases These trials are rare. phase 0 trials are tiny trials that help researchers decide if a new agent should be tested in a phase 1 trial. phase 4 trials look at long term safety and effectiveness. they occur after a new treatment has been approved and is on the market. the following shows the number of patients participating and the purpose of the. The four phases of clinical trials. early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. later phases (phase 3 and 4) compare the treatment to current standard treatments. in a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. The four phases of clinical trials represent a systematic progression of research to evaluate a potential new medical treatment thoroughly. phase 1 trials begin small, prioritizing safety in dozens of volunteers. phase 2 expands to refine the methodology and get an initial readout on efficacy. phase 3 trials are large scale, often international.

What Everybody Should Know About Clinical Trials Part 7 ођ Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. The four phases of clinical trials represent a systematic progression of research to evaluate a potential new medical treatment thoroughly. phase 1 trials begin small, prioritizing safety in dozens of volunteers. phase 2 expands to refine the methodology and get an initial readout on efficacy. phase 3 trials are large scale, often international.

Comments are closed.