What Is An Element Vs Compound In Chemistry

Unit 2 Lesson 2 6 Elements And Compounds The difference between an element and a compound is that an element is a substance made of same type of atoms, whereas a compound is made of different elements in definite proportions. examples of elements include iron, copper, hydrogen and oxygen. examples of compounds include water (h 2 o) and salt (sodium chloride nacl). As only one type of atoms makes up an element, all the properties of that atom are represented by its atom. in the case of compounds, the same type of molecules makes up the compound. 8: ability to breakdown: elements cannot be broken down by chemical reactions. compounds can be easily separated into simpler substances by chemical reactions.

Elements Vs Compounds Vs Mixtures Enthuziastic Compounds can be defined as substances consisting of 2 or more different types of elements in a fixed ratio of their atoms. when the elements combine, some individual property of the elements is lost and the newly formed compound has new properties. chemical formula: compounds are represented by their chemical formula. An element is a substance that cannot be further broken down into smaller substances by chemical means, such as hydrogen (h). the periodic table of elements lists all discovered elements. a compound is a substance made of two or more elements chemically combined and with a consistent composition, such as hydrogen and oxygen combining into water. Compounds can be classified as ionic or covalent. molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. much of the study of chemistry, however, involves looking at what happens. The difference between element and compound is part of a very important understanding in the study of chemistry. furthermore, elements and compounds are pure substances that exist in nature. most noteworthy, the main difference between element and compound is that the former comprises of same types of atoms while the later comprises of.
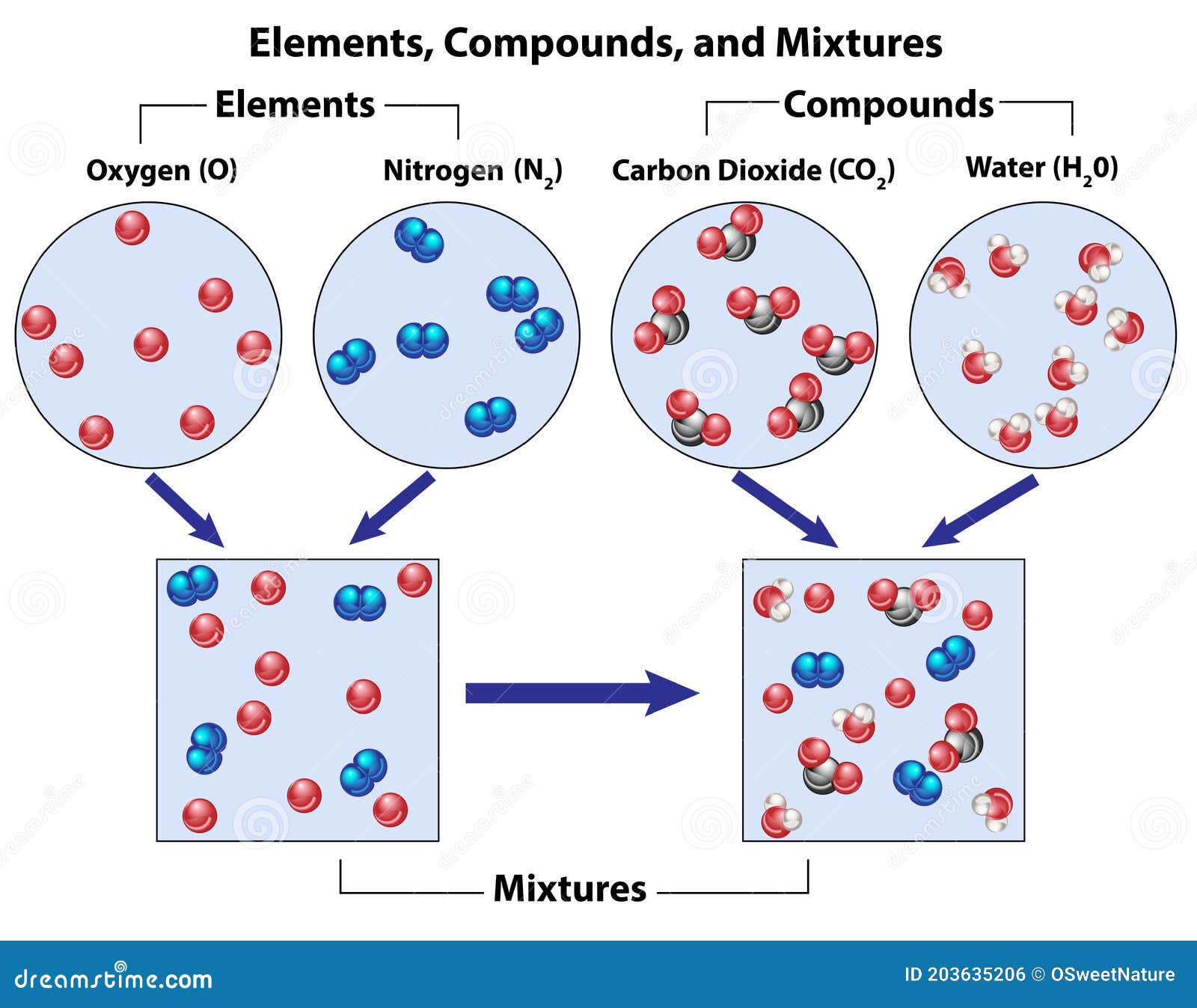
Compound Mixture Examples Hot Sex Picture Compounds can be classified as ionic or covalent. molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. much of the study of chemistry, however, involves looking at what happens. The difference between element and compound is part of a very important understanding in the study of chemistry. furthermore, elements and compounds are pure substances that exist in nature. most noteworthy, the main difference between element and compound is that the former comprises of same types of atoms while the later comprises of. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. the elements carbon and hydrogen combine to form many different compounds. one of the simplest is called methane, in which there are always four times as many hydrogen particles as carbon particles. Close. compound a pure substance made from two or more elements which are chemically bonded in a fixed ratio. and. mixtures. close. mixture when two or more compounds or elements are present.

Elements Compounds And Mixtures Mini Chemistry Learn Chemistry Online A compound is a substance that contains two or more elements chemically combined in a fixed proportion. the elements carbon and hydrogen combine to form many different compounds. one of the simplest is called methane, in which there are always four times as many hydrogen particles as carbon particles. Close. compound a pure substance made from two or more elements which are chemically bonded in a fixed ratio. and. mixtures. close. mixture when two or more compounds or elements are present.

Comments are closed.