What Is The Unit Of Rate Constant For Third Order Reaction Chemistry
Unit Of Rate Constant For Third Order Reaction The third order reaction for the above chemical reaction is given by, order = x y z. to summarise, the order of reaction can be defined as the sum of the exponents of all the reactants present in that chemical reaction. if the order of that reaction is 3, then the reaction is said to be a third order reaction. different cases in third order. If m = 1 and n = 1, the overall order of the reaction is second order (m n = 1 1 = 2). the rate law: rate = k[h 2o 2] describes a reaction that is first order in hydrogen peroxide and first order overall. the rate law: rate = k[c 4h 6]2. describes a reaction that is second order in c 4 h 6 and second order overall.

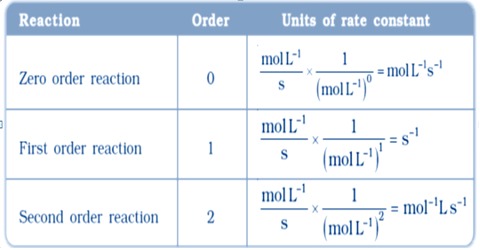
Integrated Rate Equation For Third Order Reaction Third Order From this equation, a general formula for the units of k is obtained which is: k units = m1 n · t 1. where n is the reaction order. for example, let’s say we want to determine the units of the rate constant for third order reactions. n = 3, and therefore, k units = m1 3 · t 1 = m 2 · t 1. if the time is seconds, then the units will be:. 3.3.3: reaction order. For a third order reaction, the rate constant has units of liter squared per mole squares per second (l 2 ·mol −2 ·s −1) or (m −2 ·s −1) other calculations and simulations for higher order reactions or for dynamic chemical reactions, chemists apply a variety of molecular dynamics simulations using computer software. It is important to note that rate laws are determined by experiment only and are not reliably predicted by reaction stoichiometry. the units for a rate constant will vary as appropriate to accommodate the overall order of the reaction – remembering that rate is always reported as $\frac{\delta\:concentration}{\delta\:time}$, and the units of.

Comments are closed.